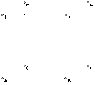Phys12
- 351
- 42
If we have a particle, say, an electron and we shoot it straight through an empty box. This box is surrounded by light sources on its two sides:

So, if you consider the above cube, if we shoot a particle in a straight line such that it crosses the face ABEF and it crosses the face HGDC through their center. And we keep an array of light sources in the faces BCGF and ADHE in the middle along the side BC and AD respectively. The light sources are high energy and hence, smaller wavelength to be able to reflect off the electron.
If we have the setup above, light would bounce off the electron on the two sides and when it does, we'll detect exactly where the electron is. Since we have these waves coming from both the directions, it wouldn't change the momentum of the particle. We could measure the position of the particle at a specific instant, do the same measurement at a different instance and then divide the change in position by change in time to get the velocity and hence, the momentum, correct?
Edit: Oops! Title didn't really match the elaboration.
So, if we can get a high enough energy light, wouldn't we be able to measure the position and the momentum at the same time with uncertainties less than h bar/2? How exactly is it the case that we're not limited by our instruments, but the inherent nature of the universe doesn't allow for such precise measurements?
So, if you consider the above cube, if we shoot a particle in a straight line such that it crosses the face ABEF and it crosses the face HGDC through their center. And we keep an array of light sources in the faces BCGF and ADHE in the middle along the side BC and AD respectively. The light sources are high energy and hence, smaller wavelength to be able to reflect off the electron.
If we have the setup above, light would bounce off the electron on the two sides and when it does, we'll detect exactly where the electron is. Since we have these waves coming from both the directions, it wouldn't change the momentum of the particle. We could measure the position of the particle at a specific instant, do the same measurement at a different instance and then divide the change in position by change in time to get the velocity and hence, the momentum, correct?
Edit: Oops! Title didn't really match the elaboration.
So, if we can get a high enough energy light, wouldn't we be able to measure the position and the momentum at the same time with uncertainties less than h bar/2? How exactly is it the case that we're not limited by our instruments, but the inherent nature of the universe doesn't allow for such precise measurements?
Attachments
Last edited: