Discussion Overview
The discussion revolves around the observation of fluctuating voltage readings during the freezing of water. Participants explore potential explanations for this phenomenon, including the effects of ice fracturing and galvanic reactions, while also discussing experimental methods and conditions.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Experimental/applied
Main Points Raised
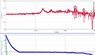
- One participant reports measuring voltage fluctuations from positive to negative while freezing water, reaching values between 0.400 V and -0.200 V.
- Another participant suggests that fractures in ice could induce voltage as it freezes.
- Some participants propose that galvanic reactions might be responsible for the observed voltages, particularly if different metals are used in probes.
- Concerns are raised about the validity of the measurements, with suggestions that the setup might be flawed or that the observed voltages are too high for typical galvanic reactions in fresh water.
- One participant mentions the need for ions in water to carry current, questioning the magnitude of the reported voltages.
- Another participant indicates that using distilled water in future experiments may yield different results.
- Discussion includes technical details about the experimental setup, including the use of temperature probes and the materials of the measuring devices.
Areas of Agreement / Disagreement
Participants express differing views on the causes of the observed voltage, with no consensus reached. Some support the idea of ice fracturing, while others emphasize the role of galvanic reactions or question the experimental setup itself.
Contextual Notes
Participants note limitations in their understanding of the phenomena, including the need for more controlled experiments and the potential influence of different materials used in the probes. There is also mention of the importance of ion concentration in water affecting voltage measurements.
Who May Find This Useful
This discussion may be of interest to those conducting experiments related to electrochemistry, phase changes in materials, or the electrical properties of water and ice.