You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
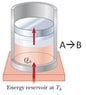
Why is this ISOTHERMAL and not ISOBARIC?
- Thread starter Farina
- Start date
-
- Tags
- Isobaric Isothermal
AI Thread Summary
The discussion centers on the distinction between isothermal and isobaric processes in the context of a piston containing an ideal gas. Participants clarify that while the process is indeed isothermal due to heat absorption from a reservoir, it cannot be classified as isobaric because the pressure does not remain constant during the gas expansion. The movement of the piston is driven by the gas pressure exceeding the weight of the piston, leading to a decrease in pressure as the gas expands. This dynamic prevents the process from being isobaric, as isobaric processes require constant pressure. Ultimately, the conversation highlights the nuances of thermodynamic definitions and their applications.
Science news on Phys.org
Haborix
- 381
- 442
Hello,
Quick question. Does the piston contain an ideal gas? If so, remember what an isotherm looks like on P vs. V diagram for an ideal gas.
I hope this helps. If not, then I'm sure someone else will come along and help you sort it out.
Quick question. Does the piston contain an ideal gas? If so, remember what an isotherm looks like on P vs. V diagram for an ideal gas.
I hope this helps. If not, then I'm sure someone else will come along and help you sort it out.
Farina
- 38
- 0
It is an Ideal Gas - thank you. I definitely see how it's an isothermal process (due to the heat reservoir). I'm still not, however, seeing how it is also not an isobaric process.
nasu
Homework Helper
- 4,466
- 918
What makes the piston to move up?
You did not describe the actual mechanism illustrated here.
You did not describe the actual mechanism illustrated here.
Farina
- 38
- 0
According to the textbook, this is simply a Carnot process (see attached image). My point is this: if the piston is free to move, then it will move however it needs to move to maintain a constant pressure - at least this is how I'm used to hearing the description of an isobaric process.
So, again, isn't this BOTH an isobaric process and isothermal process?
This seems to be quite a brain teaser.
So, again, isn't this BOTH an isobaric process and isothermal process?
This seems to be quite a brain teaser.
Attachments
nasu
Homework Helper
- 4,466
- 918
I think I understand your confusion.
You assume that the pressure of the gas is such that balances the weight of the piston.
If this were the case, the piston will not begin to move and the gas will not expand. And no heat will be absorbed, as the gas has the same temperature as the reservoir, in state A.
Imagine that the pressure in state A is larger than the weight of the piston divided by its area.
The gas pushes the piston and expands, lowering its pressure in the process. To keep its temperature constant during expansion it absorbs heat from the reservoir.
You assume that the pressure of the gas is such that balances the weight of the piston.
If this were the case, the piston will not begin to move and the gas will not expand. And no heat will be absorbed, as the gas has the same temperature as the reservoir, in state A.
Imagine that the pressure in state A is larger than the weight of the piston divided by its area.
The gas pushes the piston and expands, lowering its pressure in the process. To keep its temperature constant during expansion it absorbs heat from the reservoir.
Farina
- 38
- 0
Ah, yes - you're right, that's what I was missing. Thank you very much!
nasu
Homework Helper
- 4,466
- 918
You are very welcome.
Similar threads
- Replies
- 5
- Views
- 2K
- Replies
- 11
- Views
- 7K
- Replies
- 22
- Views
- 3K
- Replies
- 11
- Views
- 3K
- Replies
- 12
- Views
- 3K
- Replies
- 3
- Views
- 2K
- Replies
- 12
- Views
- 2K
- Replies
- 81
- Views
- 5K
- Replies
- 2
- Views
- 2K
- Replies
- 5
- Views
- 3K
Hot Threads
-
I Explain Bernoulli at the molecular level?
- Started by user079622
- Replies: 251
- Classical Physics
-
B Simple mass/scale puzzle
- Started by DaveC426913
- Replies: 206
- Classical Physics
-
I Solving a momentum problem where masses change after the collision
- Started by rdemyan
- Replies: 35
- Classical Physics
-
I What does equiprobable mean in the context of thermal motion?
- Started by Mike_bb
- Replies: 116
- Thermodynamics
-
I Topic about physics axioms, theory, laws etc..
- Started by user079622
- Replies: 93
- Classical Physics
Recent Insights
-
Insights Why Entangled Photon-Polarization Qubits Violate Bell’s Inequality
- Started by Greg Bernhardt
- Replies: 26
- Quantum Interpretations and Foundations
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 11
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 3
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 1
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 105
- General Math