adoado
- 71
- 0
The combination of potassium nitrate and sorbitol and commonly used by hobbyists as a propellant for rocket motors.
The combustion equation is given below:
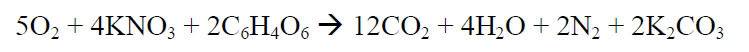
I have a few very simple questions based on the chemistry of such a reaction.
Firstly, many people do not use stoichiometric quantities of each reagent. Why is this? I thought the idea was to get the 'exact' amounts as to optimize the reaction? Or is this simply optimizing the efficiency of the utilization of the reagents into products?
Secondly, there is an oxygen term there; does this need to be available as a gas? For example, a lot of people seem to add Iron Oxide to this mix, probably to give this oxygen component. My question is, why iron oxide - can anything with an oxygen work?
Thanks for reading,
Adrian
The combustion equation is given below:
I have a few very simple questions based on the chemistry of such a reaction.
Firstly, many people do not use stoichiometric quantities of each reagent. Why is this? I thought the idea was to get the 'exact' amounts as to optimize the reaction? Or is this simply optimizing the efficiency of the utilization of the reagents into products?
Secondly, there is an oxygen term there; does this need to be available as a gas? For example, a lot of people seem to add Iron Oxide to this mix, probably to give this oxygen component. My question is, why iron oxide - can anything with an oxygen work?
Thanks for reading,
Adrian