CrimpJiggler
- 141
- 1
First one:
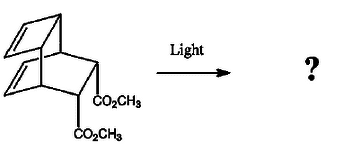
is this an intramolecular [2+2] photocycloaddition?
And this one:
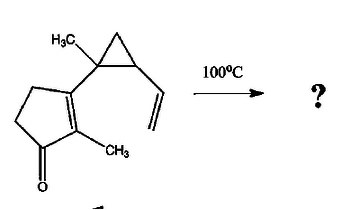
I'm fairly confused about this one. Could it be an intramolecular Alder-ene reaction? Whats confusing me most is that cyclopropyl ring up there. I know they're pretty unstable and I read that they open during electrocyclisations and stuff like that so I'm wondering if it plays a role in this reaction.
is this an intramolecular [2+2] photocycloaddition?
And this one:
I'm fairly confused about this one. Could it be an intramolecular Alder-ene reaction? Whats confusing me most is that cyclopropyl ring up there. I know they're pretty unstable and I read that they open during electrocyclisations and stuff like that so I'm wondering if it plays a role in this reaction.