Thermodynamics Definition and 1000 Threads
-
L
Teaching Thermodynamics with minimal math
Thermodynamics is an interesting subject but all too often students think of it as solving math problems. And indeed most of the problem solving involves calculations which can be quite in-depth, requiring knowledge of calculus. I have been looking for ways to deliver thermodynamics principles...- littlegreyw0lf
- Thread
- Teaching Thermodynamics
- Replies: 3
- Forum: STEM Educators and Teaching
-
J
Thermodynamics Problem: Finding Heat Transfer
I have come across this problem from this booklet: https://www.ioc.ee/~kalda/ipho/Thermodyn.pdf- Jacob White
- Thread
- Heat Heat transfer Thermodynamics
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Calculating Cp for Ideal Gases using Thermodynamic Relationships
In this particular Question according to Meyer's formula,the value of Cp should be (8.314+5) i.e. 13.314 .But that option is missing. There is another approach to this problem by finding the Adiabatic Coefficient and then finding Cp.I have no problem with that approach. But my initial doubt...- Rongeet Banerjee
- Thread
- Physics Thermodyamics Thermodynamics
- Replies: 3
- Forum: Introductory Physics Homework Help
-
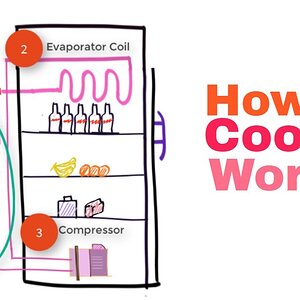
Refrigerators and Carnot Cycle (Sequence of 5 steps) #14
Refrigerators run on Carnot cycle. It is like a heat pump that operates on the reversed Carnot cycle. It utilizes the evaporation of the refrigerant to absor...- Vish
- Media item
- carnot cycle refrigerators thermodynamics
- Comments: 0
- Category: Thermodynamics
-

Calculus problem regarding Thermodynamics HW (entropy for C2H5OH at 348K)
Summary:: Seems simple but has me stumped... [Thread moved from a technical forum, so no Homework Template is shown] Hello! I am struggling to use an equation given to me. To provide some context, I am trying to work out the entropy for C2H5OH at 348K. Using provided tabulated data, the...- densephysicist
- Thread
- Calculus Physics Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
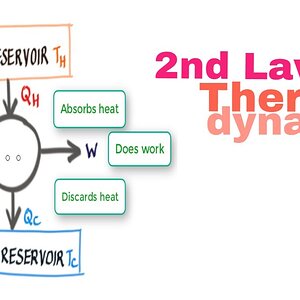
Second Law of Thermodynamics and Heat Engines #11
The Second Law of Thermodynamics is not an easy topic. However, if you understand the concept of direction of thermodynamic processes and heat engines, you w...- Vish
- Media item
- heat second law of thermodyanmics thermodynamics
- Comments: 0
- Category: Thermodynamics
-
S
Engine working between multiple temperature baths worse than Carnot
Let the new engine, NE, extract heat from a certain subset of these baths, and let heat obtained from the ##i^{\rm th}## bath be denoted by ##Q_i##, and let the heat rejected to the ##j^{\rm th}## be denoted by ##Q_j##. Let the engine perform an amount of work ##W##. Now right beside this...- Sat D
- Thread
- Carnot Carnot cycle Engine Multiple Temperature Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
A
Calculation Involving Thermodynamics, Gravitation and Fluid mechanics
I figured out stokes law equation but I am unable to proceed further- Ayesha02
- Thread
- Calculation Fluid Fluid mechanics Gravitation Mechanics Thermodynamics
- Replies: 8
- Forum: Introductory Physics Homework Help
-
B
Heat distribution in a piece of glass receiving protons
First of all, I didn't know whether to pick this subforum or the engineering/compsci one, I understand this might need to be moved to a more appropriate subforum. The general approach is fairly obvious, use implicit method to construct the tridiagonal matrix for Thomas method and solve. However...- Bqback
- Thread
- Distribution Glass Heat Heat equation Protons Thermodynamics
- Replies: 6
- Forum: Advanced Physics Homework Help
-
J
Undergrad The Physics of cooking breakfast (basic thermodynamics)
I eat eggs, ham, peppers, and a pancake for breakfast. I cook the ham and peppers at the same time. For purposes of this question, I have the eggs and the pancake figured out. Given a gas stove, a small, nonstick aluminum frying pan (with lid) and corn oil; If my goals are (in order of...- jamesson
- Thread
- Basic thermodynamics Cooking Physics Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-

I need a help on thermodynamics physics relating to Metallic Hydrogen
- Haozi02133
- Thread
- Fermi energy Hydrogen Physics Thermodynamic Thermodynamics
- Replies: 1
- Forum: Advanced Physics Homework Help
-
A
Gas - cylinder - piston problem
Summary:: Seeking explanation to classical gas - cylinder - piston problem, not the solution. Problem 1.15 from 7th edition of Introduction to Chemical Engineering Thermodynamics by Smith, Van nes and Abbot) Classical problem, given: - gas in a confined cylinder - piston with weight is placed...- akmkeng
- Thread
- Chemical engineering Cylinder Gas Piston Piston cylinder Thermodynamics Work
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
T
Thermodynamics -- Calculations for heating water up
A thermal flask with water of temperature 90c is placed out in room temperature 20c, sealed. How long before it reaches 55c? outer radius and height: 5cm, 25cm volume of water is 1litre. R = 0.5 Km2/W using this equation to solve it combining these two and integrating with respect to T...- tollf
- Thread
- Calculations Heating Thermodynamics Water
- Replies: 2
- Forum: Introductory Physics Homework Help
-
Y
Why use c_p and not c_v as specific heat - Thermodynamics
Hey all, I am working on a problem that goes like this: The cargo space of a refrigerated truck whose inner dimensions are 12 m 3 2.3 m 3 3.5 m is to be precooled from 25°C to an average temperature of 5°C. The construc- tion of the truck is such that a transmission heat gain occurs at a rate...- yjl
- Thread
- Heat Specific Specific heat Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
I
Question about thermal physics -- Ice cubes melting in water
First, I calculated the heat required for the ice to melt: Q=mLf Q=0.150×330 Q=49.5 J Then, I calculated the final temperature of the water by forming the following equation: Q=mcΔT −49.5=(0.15+0.35)×4200×(Tf −80) Tf=80.0 degrees celsius But the answer says 32 degrees Celsius.- ianc1339
- Thread
- Heat Ice Latent heat Melting Physics Thermal Thermal physics Thermodynamics Water
- Replies: 8
- Forum: Introductory Physics Homework Help
-
H
Undergrad Thermodynamics Differentiating logarithms
<mentor: change title> In thermodynamics, there is a function which, for the three variables x, y, and z, can be given as ##G = xG_x+yG_y+zG_z + N[x\ln(x) + y\ln(y)+ z\ln(z)]+E(x,y,z)## where G_x, G_y, G_z, and N are some constants and E is some arbitrarily complicated term. There is a...- Hypatio
- Thread
- Differentiating Logarithms Thermodynamics
- Replies: 11
- Forum: Differential Equations
-

Impossible thermodynamics Q :(((
- RoisinCleary
- Thread
- Impossible Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
A
Conceptual thermodynamics problem about ammonia executing a Carnot cycle
Is there a mathematical explanation for why the work done in the condenser (in process 2 to 3) is zero? I am aware that ammonia does not expand or compress in the condenser, only changes phase, but without knowing that the process takes place in a condenser and only considering the graph...- Andrew1234
- Thread
- Ammonia Carnot Carnot cycle Conceptual Cycle Thermodynamics
- Replies: 3
- Forum: Introductory Physics Homework Help
-
A
Conceptual thermodynamics question regarding specific heat ratio
The solution can be found at https://study.com/academy/answer/an-insulated-rigi... After using the two equations I can't see why (h2-h1)/(u2) should equal (T2)/(T1). Can someone explain why specific heat ratio is equal to temperature ratio?- Andrew1234
- Thread
- Conceptual Heat Ratio Specific Specific heat Thermodynamics
- Replies: 6
- Forum: Introductory Physics Homework Help
-

Undergrad The Second Law of Thermodynamics and the Stefan-Boltzmann Law
I have a question about the Second Law of Thermodynamics and the Stefan-Boltzmann law. These quotes are from http://www.ces.fau.edu/nasa/module-2/correlation-between-temperature-and-radiation.php “The Stefan-Boltzmann law, a fundamental law of physics, explains the relationship between an...- jackdale
- Thread
- Law Second law Stefan-boltzmann Stefan-boltzmann law Thermodynamics
- Replies: 6
- Forum: Thermodynamics
-
P
Thermodynamics Problem: Reversible and Irreversible Processes
Attempt at A Solution Problem 1 Reversible Process - A cylinder of ideal gas at pressure P is in mechanical equilibrium with a piston of area A driven by a spring of force F = PA and thermal equilibrium with a reservoir of temperature T. The piston is moved a small distance dx toward the...- Pendleton
- Thread
- Irreversible Irreversible processes Reversible Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Graduate Fundamental equation (thermodynamics) from Euler's equation
by substituting all values in the euler equation you get most of the terms in the fundamental equation but not (N/No)^-(c+1) How do you get this term?- VVS2000
- Thread
- Euler's equation Fundamental Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-
M
Thermodynamics Problem: Cylinder-Piston System with Friction
Hi guys, First of all I'm sorry for my bad english I'll try to be as clear as possible. I have tried to solve this problem to understand the First Law of Thermodinamics: Q+L=ΔE_t In fact I know L (in the current convention) is the work which the envirorment does on the system but I don't...- MathError
- Thread
- Friction System Thermodynamics
- Replies: 4
- Forum: Introductory Physics Homework Help
-
S
High Energy Introductory notes on AdS/CFT or black hole thermodynamics
I am looking for a book/notes on the topics mentioned in the title that would be accessible to an undergrad. I have a background in grad quantum and statistical mechanics, but most resources I found on those topics assume a familiarity with QFT, string theory, gauge theory, and general...- Silicon-Based
- Thread
- Ads/cft Black hole Hole Introductory Notes Thermodynamics
- Replies: 2
- Forum: Science and Math Textbooks
-
B
Graduate Thermodynamics: calculate thermodynamic derivative from data?
I don't understand how to use output from an NPT molecular dynamics simulation to compute a thermodynamic derivative. I need to compute this (where "d" is a partial derivative, "T" is a subscript that means, "at constant temperature," and "E" is internal energy): -(dE/dV)T I have a simulation...- bumblebee77
- Thread
- Data Derivative Molecular Simulation Thermodynamic Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-

Graduate Integration of inexact differentials in Thermodynamics
In Thermodynamics, I have seen that some equations are expressed in terms of inexact differentials, ##\delta##, instead of ##d##. I understand that this concept is introduced to point out that these differential forms are path-dependent, although I am not clear how they can be handled. So, are...- Sokolov
- Thread
- Differentials Inexact differential Integration Thermodaynamics Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-
Y
Engineering Find out the percent exergy loss
Problem, with state values, and pie chart (Fig 4.20) showing answers: ^ This shows the system in question (Kapitza Liquefaction System). Methane gas enters into the compressor (c), then goes through the first heat exchanger (HX1). Some of it (z) gets routed to the expander (exp). Afterwards...- yaro99
- Thread
- Fluids Loss Methane Percent Thermodynamics
- Replies: 17
- Forum: Engineering and Comp Sci Homework Help
-
A
Thermodynamics energy balance for control volume
Why is energy balance for a control volume dE/dt = dQ/dt-dW/dt-dm/dt(ΔH+ΔKE+ΔPE) 0 = dQ/dt-dW/dt-dm/dt(ΔH+ΔKE+ΔPE) whereas for other systems it is ΔE =Q-W-(ΔU+ΔKE+ΔPE) 0 = Q-W-(ΔU+ΔKE+ΔPE) with enthalpy, h = u +pv, replaced by only the internal energy? How is the pv term accounted for...- Andrew1234
- Thread
- Balance Control Control volume Energy Energy balance Thermodynamics Thermodynamics first law Volume
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Engineering Thermodynamics - Second Law: 2 Heat Engines Connected Between 3 Metal Blocks
Hi, I posted a similar question recently and gained some insight on these types of problems. However, I am slightly stumped on how to approach this variation of the problem. So I know that: - there is no net change in enthalpy of the blocks and the engine as the processes are reversible -...- Master1022
- Thread
- Blocks Engines Heat Heat engines Law Second law Thermodynamics
- Replies: 9
- Forum: Engineering and Comp Sci Homework Help
-
M
Thermodynamics Problem: Heat Engine Between Two Blocks
Hi, I am quite confused about how to approach this problem. I have seen variations of this problem where there is a heat engine between two blocks, but in this case the surroundings are massless, so I don't believe that approach will work here. Method: I have first started with the case that...- Master1022
- Thread
- Blocks Engine Heat Heat engine Thermodynamics Two blocks
- Replies: 16
- Forum: Introductory Physics Homework Help
-

Isothermal pressure change in a U-shaped tube
Hi, just reviewing some thermodynamics from the textbook by Sears and Salinger, having a hard time conceptualizing this one. It's an isothermal change in pressure, so the volumes of the mercury and the air both change to reach equilibrium, but if it's a "good vacuum pump", then won't the right...- obstinatus
- Thread
- Change Isothermal Pressure Thermodynamics Tube
- Replies: 4
- Forum: Introductory Physics Homework Help
-
C
Thermodynamics help please -- Air passing through a gas turbine system
Summary:: NO TEMPLATE BECAUSE THIS HOMEWORK PROBLEM WAS MISPLACED IN A REGULAR FORUM Cant do part c, using the steady flow equation I am confused how to continue. Please help! Mainly confused as to what heat transfer loss represents in the steady flow equation and where to go to find the...- clark123
- Thread
- Air Gas Gas turbine System Thermodynamics Turbine
- Replies: 17
- Forum: Engineering and Comp Sci Homework Help
-

Where do I put this Server Room's air exhaust?
Hey guys. I have a room which i want to use as a server room. the devices need to work 24/7 and they get pretty hot. so it is imperative to keep the room cool otherwise the devices will be damaged. I have an air vent to bring cold air into the room for the devices so their fans can suck in cold...- Anon_Miner
- Thread
- Air Cooling Exhaust Heat transfer Physics Server Thermo Thermodynamics
- Replies: 15
- Forum: Mechanical Engineering
-

Trouble with fluid thermodynamics and nuclear thermal rockets
Summary:: In need of help determining the exhaust velocity of a rocket nozzle given temperature and propellant molar mass Greetings and salutations! My name is Robert DeVries, world builder extraordinaire. I have come with questions in search of answers. So for the last few days I've been...- Robert DeVries
- Thread
- Fluid Fluid machanics Nuclear Physcis Rocketry Rockets Thermal Thermodyamics Thermodynamics
- Replies: 2
- Forum: Sci-Fi Writing and World Building
-
Z
Thermodynamics: find the change of internal energy, the work and Q
According to the first principle of thermodynamics: ΔU = W + Q Also noting that: W = -P⋅ΔV (Question: This P is the initial pressure or the final?) To find V2: (P1⋅V1) / T1 = (P2⋅V2) / T2 → Therefore, (P⋅V1) / T1 = [(P/5)⋅V2] / T2 → (P⋅V1) / T1 = (P⋅V2) / (5⋅T2) → V2 = (5⋅T2⋅V1) / T1...- zvwner
- Thread
- Change Energy Internal Internal energy Thermodynamics Work
- Replies: 10
- Forum: Introductory Physics Homework Help
-

Thermodynamics -- Temperature of a Heat Source?
In heat engine we define a heat source from where heat is transferred to the system, we say that heat source has a temperature ##T_h## , When we define a Carnot heat engine, the first process we have is an isothermal expansion and we say heat has to come in system through this process and here...- Rahulx084
- Thread
- Carnot cycle Entropy Heat Source Temperature Thermodynamics
- Replies: 16
- Forum: Materials and Chemical Engineering
-
T
Graduate 1st Law of thermodynamics : moving reference frame
I'd like to apply the 1st law of thermodynamics in a reference frame (RF) moving with constant velocity. We have: ##\Delta{}E = E_{in} - E_{out}## I am limiting myself to rectilinear motion. Suppose we are in a RF moving with a constant velocity ##V##. Let the system consist of a mass ##m##. The...- thinkingcap81
- Thread
- Frame Law Reference Reference frame Thermodynamics
- Replies: 8
- Forum: Thermodynamics
-
E
Graduate Confused about use of external vs internal pressure in thermodynamics
I've learned that ##W = -\int{P_{ext} dV}##, and only during a reversible/quasi-static process where ##P_{int} = P_{ext}## can we write the work done on the gas in terms of the internal pressure (and consequently use ##PV=nRT## etc. which apply to the internal gas). However, a lot of sources...- etotheipi
- Thread
- Confused Internal Pressure Thermodynamics
- Replies: 13
- Forum: Thermodynamics
-
E
Undergrad Ignoring the acceleration of a piston in thermodynamics
I was just reading a set of thermodynamics lecture notes and came across the following In most thermodynamics problems I have done, it is indeed assumed that the piston does not accelerate so we can simply equate forces on the piston. However, I don't fully understand the line of reasoning...- etotheipi
- Thread
- Acceleration Piston Thermodynamics
- Replies: 4
- Forum: Thermodynamics
-
C
High School Irreversible expansion of gas against gas
Let's say I have a liter of gas at pressure of 4 atm and T=900K. I use it to move perfect massless frictionless and insulative piston to compress a liter of 1 atm, T=300K gas. When the pressure on both sides is equal and the piston stops moving, will the temperature on both sides of the piston...- Christofer Br
- Thread
- Expansion Gas Irreversible Thermodynamics
- Replies: 10
- Forum: Thermodynamics
-
T
Graduate Adiabatic approximation in the derivation of the speed of sound
The speed of sound in a gas at temperature T is given to be ## v=\sqrt{\frac{\gamma RT}{M}}##, where ##\gamma## is the adiabatic exponent, R is the gas constant and M is the molar mass of the gas. In deriving this expression, we assumed that the compression and expansion processes were so fast...- Terry Bing
- Thread
- Adiabatic Adiabatic process Approximation Derivation Sound Speed Speed of sound Thermodynamics
- Replies: 6
- Forum: Mechanics
-
F
Graduate Temperatures of the hot source and cold sink in a heat engine
Hi, I was just wondering about the efficiency of a cycle that is not Carnot cycle. In that case one should use \eta = 1-\left|\frac{Q_{\rm out}}{Q_{\rm in}}\right|, where Q_{\rm in} and Q_{\rm out} are the amounts of heat absorbed and released during the cycle. For instance, I guess that in...- FranzDiCoccio
- Thread
- Cold Cycle Efficiency Engine Heat Heat engine Hot Source Thermodynamics
- Replies: 2
- Forum: Thermodynamics
-
H
Thermodynamics: Why does salt lower the melting point of ice?
There was a question on "Why salt lower the freezing point of water?"I found the following answer."Thermodynamics teaches that a loss of entropy can be overcome by a gain in so called enthalpy". The loss of entropy by freezing the solution canbe over come at temperature much below 0 degree C...- hariharan venkatasu
- Thread
- Ice Melting Melting point Point Salt Thermodynamics
- Replies: 21
- Forum: Chemistry
-
C
Thermodynamics - Change in temperature for heated slabs of steel
Just started this topic so I'm not sure if this is the correct way to solve this, any help would be appreciated. mass of object x change in temp x specific heat capacity= heat change in temp= heat/(mass of object x specific heat capacity) Mass= 700kg Specific heat capacity of steel= 0.42kJ/kgC...- CJoy
- Thread
- Change Steel Temperature Thermodynamics
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Thermodynamics problem involving engine efficiency
Q2: An engine has power of 26.5 kW, needs 9 kg coal during an hour for energy. The heat capacity of coal is 7800 cal/g. Define the engine’s efficiency. Qh= mcΔT = 9000x7800xΔT ( stuck) P=26.5kW(is this the power output of the engine in an hour?) If only I could find ΔT, thenI would be able to...- Stephen Bulking
- Thread
- Efficiency Engine Engine efficiency Thermodynamics
- Replies: 20
- Forum: Introductory Physics Homework Help
-

Finding Isentropic Enthelpy, knowing Isentropic Entropy
A short background: My question focuses solely on the part of the refrigeration cycle to do with the compressor, where the cycle begins. The first state is before the refrigerant enters the compressor, and the second state is after the refrigerant leaves the compressor. My goal is to obtain...- WhiteWolf98
- Thread
- Entropy Isentropic Thermodynamics
- Replies: 3
- Forum: Introductory Physics Homework Help
-
A
Graduate Does classical statistical physics predict newer things vs. thermodynamics?
I'm wondering if the passage from a classical thermodynamic theory, i.e. which does not resort to an atomistic theory and methods of probability and statistics, to classical (i.e. non-quantum) statistical mechanics, led to new discoveries and especially if it was able to explain properties of...- Aidyan
- Thread
- Classical Physics Statistical Statistical physics Thermodynamics
- Replies: 4
- Forum: Thermodynamics
-

Entropy and the Laws of Thermodynamics
Hello everyone, I have to write a paper about entropy and how it relates to the laws of thermodynamics, energy, and work. I have taken a deductive approach starting from the zero-th law to the second law of thermodynamics as follows. Entropy is the disorder of a system (Class Video, 2019)...- Physics345
- Thread
- Entropy Laws Thermodynamics
- Replies: 3
- Forum: Introductory Physics Homework Help
-
C
Is Specific impulse indicative of performance as a gun propellant?
Black powder has specific impulse of around 80s, while rocket candy has up to 130s of specific impulse. Does that mean I could replace the propellant in a BP cartridge with 80/130 of the weight in rocket candy and obtain the same performance in an idealized gun? (as in without considering...- Christofer Br
- Thread
- Gun Impulse performance Propellant Rockets Specific Thermodynamics
- Replies: 1
- Forum: Aerospace Engineering
-
D
Entropy and the second law of thermodynamics
- denniszhao
- Thread
- Entropy Law Second law Thermodynamics
- Replies: 13
- Forum: Introductory Physics Homework Help