ScrubsFan
- 15
- 0
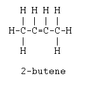
When 2-butene reacts with hydrogen chloride gas, only one product is detected, whereas when 1-butene reacts similarly, two products are usually found. Explain this.
I know that when 2-butene reacts with hydrogen chloride that the bond breaks and the Cl2 adds on one atom to each carbon in a addition reaction (resulting in 2-chlorobutane). I know the double bond will be between carbons 1 and 2 now in 1-butene, but I don't know how, and why there is two products from this.
Can someone explain this please.
I know that when 2-butene reacts with hydrogen chloride that the bond breaks and the Cl2 adds on one atom to each carbon in a addition reaction (resulting in 2-chlorobutane). I know the double bond will be between carbons 1 and 2 now in 1-butene, but I don't know how, and why there is two products from this.
Can someone explain this please.