krzywan901
- 2
- 0
Hi everyone,
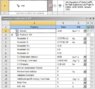
I have a problem. I need doing simulation in AnSys about explosion methane (as gas), unfortunately program has not such material and I do not know explosion parameters of methane (8.5% in air). I am enclosing picture with parameter of TNT (AnSys has material TNT), and does someone know about what parameters should has methane gas and could you share with me?
Best regards
I have a problem. I need doing simulation in AnSys about explosion methane (as gas), unfortunately program has not such material and I do not know explosion parameters of methane (8.5% in air). I am enclosing picture with parameter of TNT (AnSys has material TNT), and does someone know about what parameters should has methane gas and could you share with me?
Best regards