dRic2
Gold Member
- 887
- 225
Hi, in the lecture notes my professor gave us, it is stated that, due to Kramers theorem, the energy in a band must satisfy this condition:
$$E(-k) = E(k)$$
But, judging from actual pictures of band structures I don't find this condition to be true. Here's a (random) picture
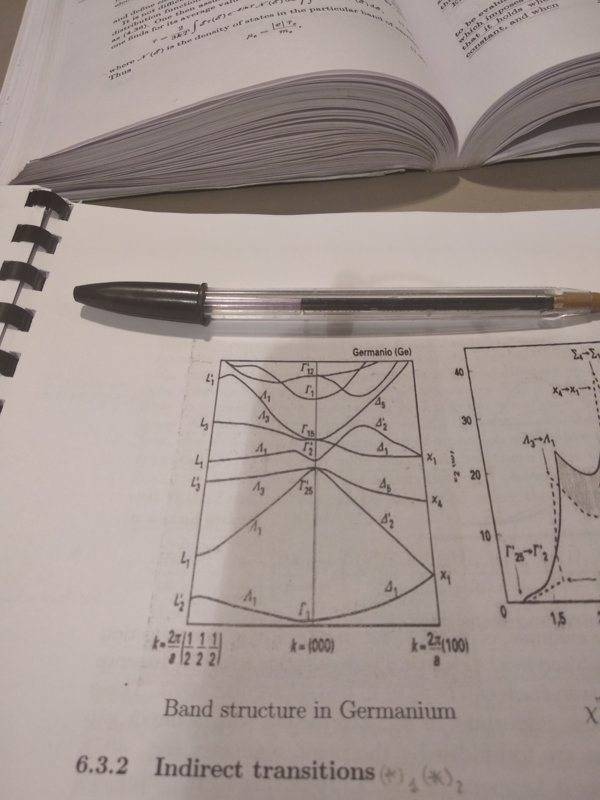
I guess it looks "kind of" symmetric in the lower bands, but I wouldn't certainly call it that way.
$$E(-k) = E(k)$$
But, judging from actual pictures of band structures I don't find this condition to be true. Here's a (random) picture
I guess it looks "kind of" symmetric in the lower bands, but I wouldn't certainly call it that way.