MrB3nn
- 16
- 0
Hello,
This is not a 'homework problem' but I think it fitted more correctly in this thread than other more advanced ones.
Basically, I am a first year undergraduate who did not study Chemistry at A-level. I am quite a serious student and I am trying to increase my background understanding of a few aspects of inter-atomic and molecular bonds. I have tried to accumulate a kind of broad understanding of the goings on on a molecular within matter. I find textbooks either don't answer the questions I have or they are far too advanced to even comprehend at my level. I really need someone to read my idea of what is happening then to tell me if it's correct or not. I also have some questions and would appreciate it if anyone could answer them.
So far, as I understand it:
There are different types of processes responsible for the forming of chemical bonds between elements.
Some of the most important are: Covalent, Ionic and Polar-Covalent.
I understand the type of bond formed between two atoms depends a lot on the electronegativities of the two atoms. Quantitively, if the difference in electronegativity on the Pauling scale is >2.0 we have a largely ionic bond. Between 0.9 and 2.0 we have polar-covalent and less than 0.9 is covalent.
I understand ionic bonds are non-saturated and can therefore exist between more than one pair of atoms, whereas covalent are saturated.
My first question is, if atoms are initially largely neutral (except for charge distribution fluctuations), why do covalent bonds form? I have heard this "because one atom wants to lose its valence electron and another wants to gain one" but this cannot satisfy me. Since the atoms are both initially neutral I imagine it has more to do with energy or quantum mechanics but I'm not sure. I am quite happy to be told the theory is above my level as long as I have some sort of incling as to what's involved.
I also understand that there are various inter-molecular forces at play in matter. Most of these are electric in nature i.e. Hydrogen bonds, Van Der Waals forces. I have a brief understanding of what each of these involves at a basic level but my knowledge lacks a link to a real sample of matter.
In a real piece of matter i.e. a solid, what is holding the molecules in a lattice, dispersion forces? Dipole-dipole forces, depending on the type of molecules? I don't understand which actual physical force is responsible.
Am I right in thinking in a liquid, dispersion forces play more of a role in determining the structure?
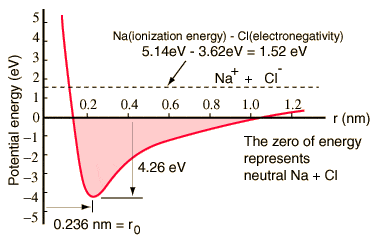
I have one last question, in a graph like this one, which forces is this taking into account? Do these diagrams take into account these Van der Waals forces? I understand the repulsion at short separation is largely due to the Pauli exclusion principle.
Thanks for your time and I hope you can shed some light on this subject for me.
This is not a 'homework problem' but I think it fitted more correctly in this thread than other more advanced ones.
Basically, I am a first year undergraduate who did not study Chemistry at A-level. I am quite a serious student and I am trying to increase my background understanding of a few aspects of inter-atomic and molecular bonds. I have tried to accumulate a kind of broad understanding of the goings on on a molecular within matter. I find textbooks either don't answer the questions I have or they are far too advanced to even comprehend at my level. I really need someone to read my idea of what is happening then to tell me if it's correct or not. I also have some questions and would appreciate it if anyone could answer them.
So far, as I understand it:
There are different types of processes responsible for the forming of chemical bonds between elements.
Some of the most important are: Covalent, Ionic and Polar-Covalent.
I understand the type of bond formed between two atoms depends a lot on the electronegativities of the two atoms. Quantitively, if the difference in electronegativity on the Pauling scale is >2.0 we have a largely ionic bond. Between 0.9 and 2.0 we have polar-covalent and less than 0.9 is covalent.
I understand ionic bonds are non-saturated and can therefore exist between more than one pair of atoms, whereas covalent are saturated.
My first question is, if atoms are initially largely neutral (except for charge distribution fluctuations), why do covalent bonds form? I have heard this "because one atom wants to lose its valence electron and another wants to gain one" but this cannot satisfy me. Since the atoms are both initially neutral I imagine it has more to do with energy or quantum mechanics but I'm not sure. I am quite happy to be told the theory is above my level as long as I have some sort of incling as to what's involved.
I also understand that there are various inter-molecular forces at play in matter. Most of these are electric in nature i.e. Hydrogen bonds, Van Der Waals forces. I have a brief understanding of what each of these involves at a basic level but my knowledge lacks a link to a real sample of matter.
In a real piece of matter i.e. a solid, what is holding the molecules in a lattice, dispersion forces? Dipole-dipole forces, depending on the type of molecules? I don't understand which actual physical force is responsible.
Am I right in thinking in a liquid, dispersion forces play more of a role in determining the structure?
I have one last question, in a graph like this one, which forces is this taking into account? Do these diagrams take into account these Van der Waals forces? I understand the repulsion at short separation is largely due to the Pauli exclusion principle.
Thanks for your time and I hope you can shed some light on this subject for me.