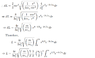fisher garry
- 63
- 1
the spin, S, for an electron is
$$\frac{\hbar}{2}=5.27 \cdot 10^{-35} $$
$$\frac{2MR^2 \omega}{5}=\frac{2MRv}{5}$$
It is said that the speed of an electron is 2200 km per second and can be calculated in classical manners from electrostatic and accelerating forces on the electron
from (1.11) the spin is $$\frac{2MRv}{5}=\frac{2 \cdot 9.1 \cdot 10^{-31} \cdot 5.291 \cdot 10^{-11} \cdot 2200 \cdot 10^{3}}{5}=4.23 \cdot 10^{-35} $$
It is almost the same. Is this just from approximation errors? My problem is also that I have used bohr radius which is calculated by quantum physics rules not classical rules as far as I know? And since this is based on moment of inertia of a sphere one must use an R that is larger then the minimum R which I use the only problem is that electron cloud of the electron is not evenly distributed so where would one set this R? And since we are looking for an outer R and we are not looking for v in the outer R we can use an average v which could lead to a more precise relation of Plancks constant?
Last edited: