RandiSS
- 5
- 1
- Homework Statement
- A gas expands from I to F in the figure. The energy added to the gas by heat is 486 J when the gas goes from I to F along the diagonal path.
1. What is the change in internal energy of the gas?
Answer in units of J.
2. How much energy must be added to the gas by heat for the indirect path IAF to give the same change in internal energy? Answer in units of J.
- Relevant Equations
- ΔU = Q + W
W=pΔV
Not a solution. This is the graph provided.
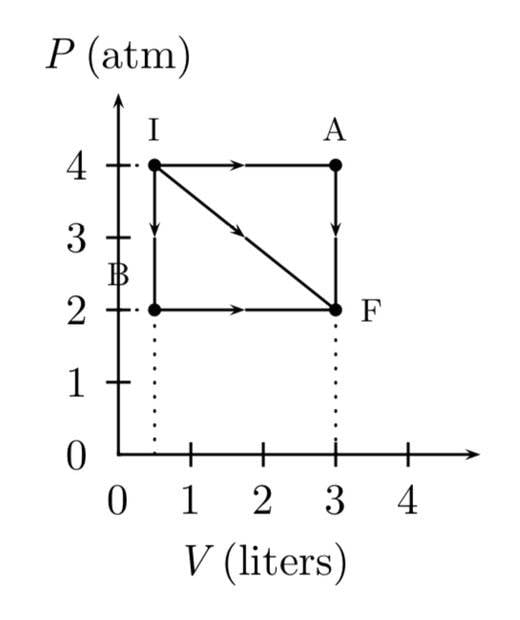
I think I start with finding the magnitude of the IF vector but I’m not sure. And I don’t know where to go from there.
I think I start with finding the magnitude of the IF vector but I’m not sure. And I don’t know where to go from there.