- #1
DrFreeman
- 2
- 0
I need help with figuring out how to devise an equation that I'm going to plug into an algorithm for my software.
These are the components:
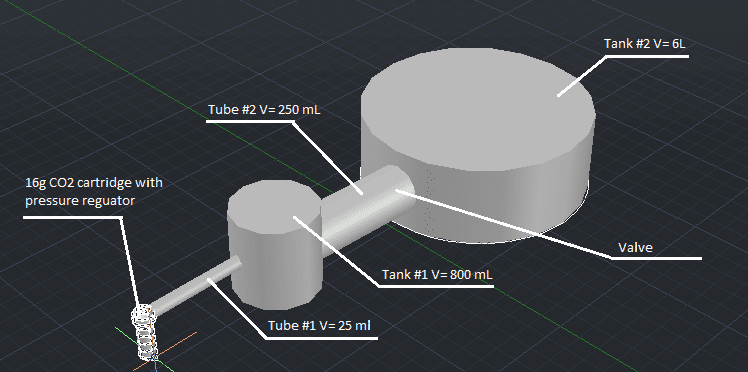
CO2 cartridge pressure regulator output should be around 200 psi. Tank #1 has a volume of 800 mL, pressure is around 15 psi. There are no valves between pressure regulator and the first tank. Pressure in tank #2 is about 7 psi (soft vacuum). There is a valve between first and second tank, right at the entry point for tank #2. The question is, what will happen when the valve between tank #1 and tank #2 is opened and the CO2 from the cartridge released at the same time? I need to calculate the amount of suction created at the valve. Also, how much time will be needed until CO2 is redistributed equally throughout the system?
These are the variables: first tank volume, second tank volume, radius and length of first pipe, radius and length of second pipe and pressure regulator output value. The variables for pressure in tanks before gas release can be taken as constant values I described above (15 psi and 7 psi). Also, system will always be running at a room temperature of 25 degrees Celsius. Values for V1 and V2 above are taken as placeholders, just for this example. The goal is to plug in these 7 variables (Rout, V1, V2, r1, h1, r2, h2) into a function and get resulting suction at tank #2 entry point with an approximate gas redistribution time.
If something above is fundamentally wrong, or if I missed anything important, please let me know. I have no experience with dealing with these kind of issues.
These are the components:
CO2 cartridge pressure regulator output should be around 200 psi. Tank #1 has a volume of 800 mL, pressure is around 15 psi. There are no valves between pressure regulator and the first tank. Pressure in tank #2 is about 7 psi (soft vacuum). There is a valve between first and second tank, right at the entry point for tank #2. The question is, what will happen when the valve between tank #1 and tank #2 is opened and the CO2 from the cartridge released at the same time? I need to calculate the amount of suction created at the valve. Also, how much time will be needed until CO2 is redistributed equally throughout the system?
These are the variables: first tank volume, second tank volume, radius and length of first pipe, radius and length of second pipe and pressure regulator output value. The variables for pressure in tanks before gas release can be taken as constant values I described above (15 psi and 7 psi). Also, system will always be running at a room temperature of 25 degrees Celsius. Values for V1 and V2 above are taken as placeholders, just for this example. The goal is to plug in these 7 variables (Rout, V1, V2, r1, h1, r2, h2) into a function and get resulting suction at tank #2 entry point with an approximate gas redistribution time.
If something above is fundamentally wrong, or if I missed anything important, please let me know. I have no experience with dealing with these kind of issues.