- #1
KedarMhaswade
- 35
- 6
- TL;DR Summary
- How do we create a partial vacuum to boil water at about 60℃?
Water (any liquid) in a closed container boils when the vapor pressure of water (a property that depends on its temperature) equals the surrounding pressure. The vapor pressure of water at 60℃ is about ##1.99\times10^{4}## which is roughly 150 mm Hg.This principle is so simple, and yet, it is rather difficult to demonstrate effectively at home (with cheap equipment the links to which I have posted to below). I have thought of doing this experiment in the following (perhaps simplistic) manner (see the Figure).
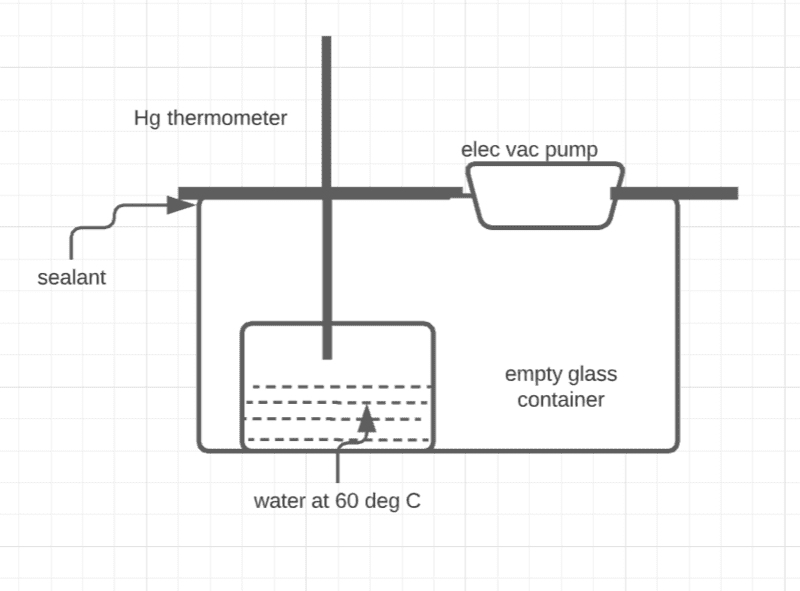
- Take a glass container and place a beaker of water at about 60℃ in it.
- Insert a thermometer and an electric vacuum pump in a lid and seal that lid to the container with some sealant like M-seal.
- Operate the pump to create partial vacuum.
- I believe it should work, but are there any obvious flaws in my setup?
- Any tips to maximize the probability of success?