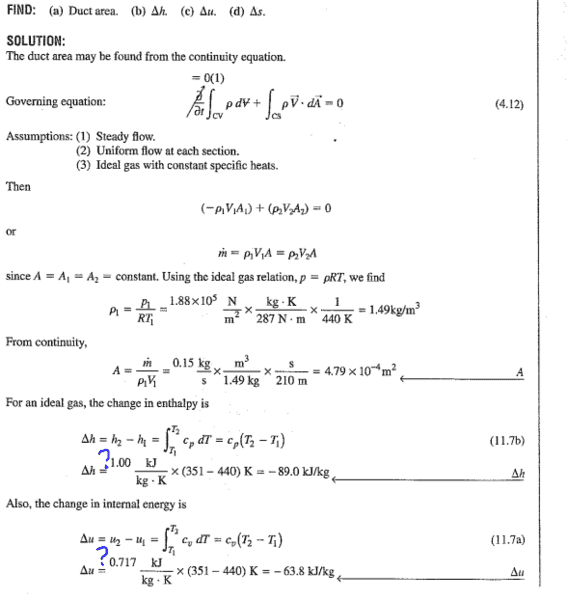
Discussion Overview
The discussion revolves around understanding the values of molar heat capacities at constant pressure (cp) and constant volume (cv) as presented in example problem 11.1. Participants are trying to clarify the derivation of these values and their relation to the ideal gas law and specific equations.
Discussion Character
- Technical explanation
- Debate/contested
Main Points Raised
- One participant questions the source of the values cp=1.00 and cv=0.717, seeking clarification on their derivation.
- Another participant provides a calculation: (3.5)(8.314)/29=1.00, but does not explain the origin of the numbers used.
- A subsequent post reiterates the calculation but also asks for clarification on the values used for cv.
- A different participant introduces a question regarding the relationship between molar heat capacities and the ideal gas constant, suggesting a theoretical exploration of the topic.
Areas of Agreement / Disagreement
Participants do not appear to reach a consensus on the derivation of cp and cv values, with multiple questions and calculations presented without resolution.
Contextual Notes
The discussion includes assumptions about the ideal gas behavior and the specific conditions under which the heat capacities are defined, but these assumptions are not fully explored or agreed upon.
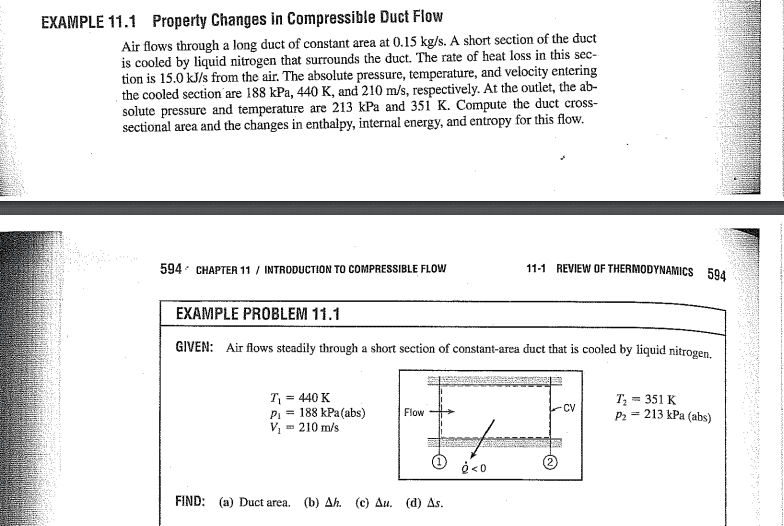 Their solution:
Their solution: