dislect
- 163
- 0
Hi guys,
Heard an interview question from a friend, the guy asked him what happens to a disk with internal radius r and external R such as the following:
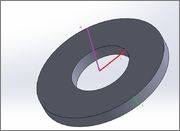
When heated.
He told me the answer is that the internal radius gets smaller and the external gets bigger.
Can anyone help me understand why (does it come on the expense of the disk thickness - t?) and put it into mathematical terms?
Thanks!
Heard an interview question from a friend, the guy asked him what happens to a disk with internal radius r and external R such as the following:
When heated.
He told me the answer is that the internal radius gets smaller and the external gets bigger.
Can anyone help me understand why (does it come on the expense of the disk thickness - t?) and put it into mathematical terms?
Thanks!
Last edited: