EL AALLAOUI Najla
- 8
- 0
How did you find PF?: via Google
Hello everyone, I hope you are doing well?
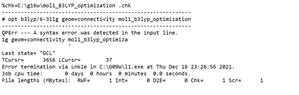
Recently, I’ve been trying to calculate the optimization and Uv spectra of organometallic compounds using the Gaussian software .it’s my first time for the computational chemistry and I am inexperienced . When I run the measure using B3LYP/6-311G basis set I have an error in my calculation and I don't know what the problem is. So, I would be grateful if you show me how to solve it and could you give me the advice about the proper basis set and functional to calculate the MO energies knowing that I have C,H,N and Cu as atoms . And I attach the message received.
Thank you very Much
Hello everyone, I hope you are doing well?
Recently, I’ve been trying to calculate the optimization and Uv spectra of organometallic compounds using the Gaussian software .it’s my first time for the computational chemistry and I am inexperienced . When I run the measure using B3LYP/6-311G basis set I have an error in my calculation and I don't know what the problem is. So, I would be grateful if you show me how to solve it and could you give me the advice about the proper basis set and functional to calculate the MO energies knowing that I have C,H,N and Cu as atoms . And I attach the message received.
Thank you very Much