- #1
member 731016
- Homework Statement
- Please see below
- Relevant Equations
- Please see below
For this,
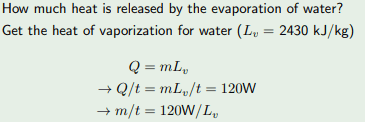
Dose anybody please know where they got the 120W from?
Many thanks!
Dose anybody please know where they got the 120W from?
Many thanks!
Last edited by a moderator: