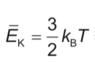youmei0426
- 18
- 0
Homework Statement
Homework Equations
pV=nRT
The Attempt at a Solution
I know the volume of the cylinder, which is Al. So I plugged this into the ideal gas law formula, and got answer B. However, the correct answer should be D. I see the Boltzmann's constant there in the equation, and I do know an equation which contains the constant, but I'm not sure how to incorporate it into the ideal gas law.. Thanks for your help in advance!