bbbl67
- 216
- 21
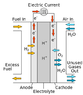
I understand that there is some research going on into gasoline (i.e. petrol) fuel cells (as opposed to the more typical hydrogen or alcohol fuel cells). How much energy can be released through a gasoline fuel cell, as opposed to simply burning it in an internal combustion engine? Will more energy be released or less than combustion? Also how much weight do fuel cell apparatuses usually weigh? Compared to an IC engine of course. Just looking for ballpark figures.
Just wondering if fuel cells would be more practical power sources for electric engines than batteries? And if so, how much efficiency can be expected? The reason I'm asking about gasoline fuel cells is because the infrastructure for gasoline already exists throughout the world. I'm pretty convinced that an electric engine is miles above an IC engine in terms of both performance and efficiency, so having cars driven by electric motors is the way to go from here on in, just it's a matter of whether batteries or fuel cells should be the power source?
Just wondering if fuel cells would be more practical power sources for electric engines than batteries? And if so, how much efficiency can be expected? The reason I'm asking about gasoline fuel cells is because the infrastructure for gasoline already exists throughout the world. I'm pretty convinced that an electric engine is miles above an IC engine in terms of both performance and efficiency, so having cars driven by electric motors is the way to go from here on in, just it's a matter of whether batteries or fuel cells should be the power source?