Klacid
- 2
- 0
Hey guys i wanted to know how make some chemicals when i have an equation, for example:
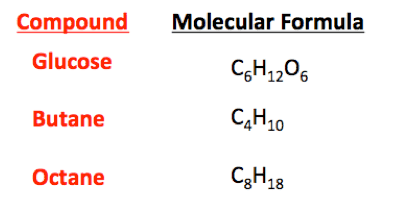
First of all, you don't have any equations in the graphic above. These are called the molecular formulas for the various compounds shown.Klacid said:Hey guys i wanted to know how make some chemicals when i have an equation, for example:
