crclayton
- 3
- 0
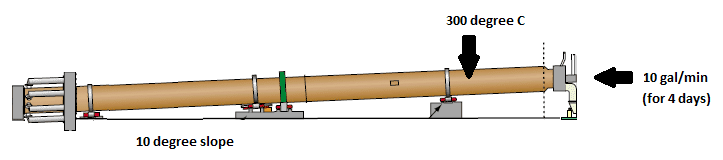
If I throw 10 gallons/minute of cold water for 4 days into an oven that's 300°C Celcius and 300ft long sloped at an angle of 10°, how far would the water make it down the oven before completely evaporating?
Even if you can't solve this directly, I'd love some advice or recommendations of how to approach the problem. I'm an EE student on an internship and this is totally out of my area of expertise.
Thanks in advance.
Even if you can't solve this directly, I'd love some advice or recommendations of how to approach the problem. I'm an EE student on an internship and this is totally out of my area of expertise.
Thanks in advance.