WMDhamnekar
MHB
- 381
- 30
Hi,
Answer given is $E_n=29.4 Joules$ Here is the question.
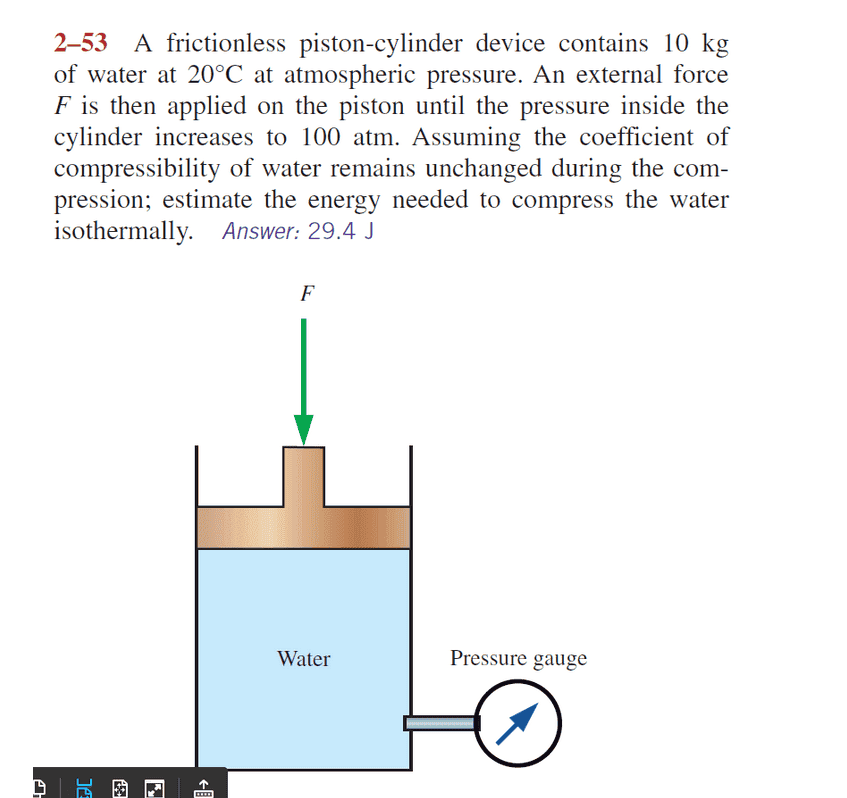
Answer provided by the Chemistry math expert/Professor is as follows but it is different from the answer given. How is that?
Compressibility is the fractional change in volume per unit increase in pressure. For each atmosphere increase in pressure, the volume of water would decrease 46.4 parts per million.
I'll pick a shape for the device, calculate distance traveled and force required, and use $work = force \times distance.$
with 100 atm, volume would decrease by 4640 PPM or by a factor of 0.00464
10 kg of water is about 10 liters or $0.01 m^3$
$100 atm = 1.013e7 Pa$ or $1.013e7 N/m^2$
assume a cube shape, height is $\sqrt[3]{0.01 m^3} = 0.2154435 m$ and base area is$ 0.0464159 m^2$ (coincidence that "464" appears as two different values)
force on piston is $1.013e7 N/m^2 \times 0.0464 m^2 = 470000 N$
that change in volume causes what change in height
new volume $= 0.01 m^3 – 0.01 m^3(0.00464) = 0.00995 m^3$
which has a height of $\frac{0.00995 m^3}{0.0464159 m^2} = 0.2143663 ,$
change is 0.2154435 – 0.2143663 = 0.00108 m
energy = Fd = (470000 N)(0.00108 m) = 506 JIs temperature of water $20^\circ C$ to be considered?
Answer given is $E_n=29.4 Joules$ Here is the question.
Answer provided by the Chemistry math expert/Professor is as follows but it is different from the answer given. How is that?
Compressibility is the fractional change in volume per unit increase in pressure. For each atmosphere increase in pressure, the volume of water would decrease 46.4 parts per million.
I'll pick a shape for the device, calculate distance traveled and force required, and use $work = force \times distance.$
with 100 atm, volume would decrease by 4640 PPM or by a factor of 0.00464
10 kg of water is about 10 liters or $0.01 m^3$
$100 atm = 1.013e7 Pa$ or $1.013e7 N/m^2$
assume a cube shape, height is $\sqrt[3]{0.01 m^3} = 0.2154435 m$ and base area is$ 0.0464159 m^2$ (coincidence that "464" appears as two different values)
force on piston is $1.013e7 N/m^2 \times 0.0464 m^2 = 470000 N$
that change in volume causes what change in height
new volume $= 0.01 m^3 – 0.01 m^3(0.00464) = 0.00995 m^3$
which has a height of $\frac{0.00995 m^3}{0.0464159 m^2} = 0.2143663 ,$
change is 0.2154435 – 0.2143663 = 0.00108 m
energy = Fd = (470000 N)(0.00108 m) = 506 JIs temperature of water $20^\circ C$ to be considered?
Last edited: