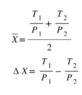You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How to find the change from a differentials problem (thermal)
- Thread starter Clara Chung
- Start date
-
- Tags
- Change Differentials Thermal
AI Thread Summary
The discussion revolves around the calculation of changes in entropy between two thermodynamic systems with differing temperatures and pressures. Participants express confusion over the signs in the equations used for entropy changes, questioning whether the textbook or their interpretations are correct. The conversation highlights the importance of understanding internal energy as a property rather than a quantity in transit, with suggestions that the problem lacks clarity. There is also a debate on the applicability of certain equations to the problem, emphasizing that they may only be valid for closely neighboring equilibrium states. The participants ultimately seek to clarify the relationship between temperature, pressure, and entropy in the context of ideal gases.
Physics news on Phys.org
- 5,917
- 3,110
To me it looks like they got the signs wrong. ## \\ ## ## \Delta S_2=(\frac{1}{T_2}) \Delta U+ (\frac{p_2}{T_2}) \Delta V ##. ## \\ ## Meanwhile ## \Delta S_1=- [(\frac{1}{T_1})\Delta U+(\frac{p_1}{T_1}) \Delta V ] ##. ## \\ ## Then ## \Delta S _{system} =\Delta S_1+\Delta S_2 ## ## \\ ## @Chestermiller Did I get the sign wrong, or did the book get the sign wrong?
- 23,704
- 5,917
What is it supposed to mean for internal energy to be transferred from system 1 to system 2? Internal energy is supposed to be a physical property of a material, not a quantity of energy in transit? Do they mean heat rather than internal energy? I have major problems with this question statement. This is the kind of thing that confuses students to no end.Charles Link said:To me it looks like they got the signs wrong. ## \\ ## ## \Delta S_2=(\frac{1}{T_2}) \Delta U+ (\frac{p_2}{T_2}) \Delta V ##. ## \\ ## Meanwhile ## \Delta S_1=- [(\frac{1}{T_1})\Delta U+(\frac{p_1}{T_1}) \Delta V ] ##. ## \\ ## Then ## \Delta S _{system} =\Delta S_1+\Delta S_2 ## ## \\ ## @Chestermiller Did I get the sign wrong, or did the book get the sign wrong?
Chet
- 5,917
- 3,110
What I think you are saying, @Chestermiller is even if a quantity of heat ## \Delta Q ## goes from system 1 to system 2, there is no guarantee that it goes into the ## \Delta U ## term=it could also go into the ## p \Delta V ## term, so the problem really lacks definition. Did I understand your comments accurately?Chestermiller said:What is it supposed to mean for internal energy to be transferred from system 1 to system 2? Internal energy is supposed to be a physical property of a material, not a quantity of energy in transit? Do they mean heat rather than internal energy? I have major problems with this question statement. This is the kind of thing that confuses students to no end.
Chet
- 23,704
- 5,917
I think what the question meant to analyze was a case where you have two systems in contact with one another having different temperatures and pressures, but with a constant total volume and a constant total internal energy. The combined system is insulated and rigid on the outside. The barrier between the chambers is released and allowed to move freely, and the system is then allowed to re-equilibrate. The idea is to determine the change in entropy from the initial to the final state, and to show that the only way entropy doesn't increase is that if the initial temperatures and the initial pressures match. For ideal gases, this problem can be analyzed without too much trouble. But I strongly disagree with using Eqn. 14-24 to analyze this because that equation applies only to closely neighboring equilibrium states of a system, and the equilibrium states would not be closely neighboring for each of the two chambers involved.
If the OP would like to analyze the ideal gas problem I described, I would be glad to help.
If the OP would like to analyze the ideal gas problem I described, I would be glad to help.
Last edited:
- 23,704
- 5,917
Clara, Is your "like" an indication that you would like me to help you solve this problem correctly (rather than the way your book does it)?
Clara Chung
- 300
- 13
Dear Chestermiller,Chestermiller said:Clara, Is your "like" an indication that you would like me to help you solve this problem correctly (rather than the way your book does it)?
I am thinking about it
Clara Chung
- 300
- 13
I don't understand why the equation only applies to closely neighboring equilibrium states, could you explain a bit more?Chestermiller said:But I strongly disagree with using Eqn. 14-24 to analyze this because that equation applies only to closely neighboring equilibrium states of a system, and the equilibrium states would not be closely neighboring for each of the two chambers involved.
George Jones
Staff Emeritus
Science Advisor
Gold Member
- 7,643
- 1,601
Clara Chung said:I don't understand why the equation only applies to closely neighboring equilibrium states
Are you familiar (as covered in something like Calc 3) with the Taylor expansion about ##\left( x_0 , y_0 \right)## of a function of two variables, ##f \left( x , y \right)##?
- 23,704
- 5,917
The thermodynamic functions U and S apply only to thermodynamic equilibrium states.Clara Chung said:I don't understand why the equation only applies to closely neighboring equilibrium states, could you explain a bit more?
Clara Chung
- 300
- 13
Yes, f(x,y) = f(x0, y0) + ∂f/∂x (x-x0) + ∂f/∂y (y-y0) + error term, but what are the function and variables?George Jones said:Are you familiar (as covered in something like Calc 3) with the Taylor expansion about ##\left( x_0 , y_0 \right)## of a function of two variables, ##f \left( x , y \right)##?
Clara Chung
- 300
- 13
Chestermiller said:I think what the question meant to analyze was a case where you have two systems in contact with one another having different temperatures and pressures, but with a constant total volume and a constant total internal energy. The combined system is insulated and rigid on the outside. The barrier between the chambers is released and allowed to move freely, and the system is then allowed to re-equilibrate. The idea is to determine the change in entropy from the initial to the final state, and to show that the only way entropy doesn't increase is that if the initial temperatures and the initial pressures match. For ideal gases, this problem can be analyzed without too much trouble. But I strongly disagree with using Eqn. 14-24 to analyze this because that equation applies only to closely neighboring equilibrium states of a system, and the equilibrium states would not be closely neighboring for each of the two chambers involved.
If the OP would like to analyze the ideal gas problem I described, I would be glad to help.
If I use solve it as an ideal gas, let the system with T1 , P1, V1 and n1 be system 1.
Let the system with T2 , P2, V2 and n2 be system 2.
Let the final temperature be Tf and final pressure be Pf.
I try to find the change of entropy between two equilibrium states, so if change in entropy = 0, the two equilibrium states will be equivalent?(I am not sure about this statement.
The internal energy of the system 1 and system 2 remains the same, i.e. n1 T1 + n2 T2 = (n1 + n2 )Tf.
Consider system 1,
the first reversible step will be an isothermal expansion from V1 to V1 + V2,
the entropy change in this reversible process is n1 R ln (V1 + V2 / V1).
the second step will be an isochoric heating to slowly increase the temperature from T1 to Tf,
dQrev = Cv dT
ΔS1=Cv ln (Tf / T1) + n1 R ln (V1 + V2 /V1)
Similarly for system 2,
so the total change in entropy is:
ΔS1 + ΔS2
= Cv ln (Tf / T1) + n1 R ln ((V1 + V2) /V1) + Cv ln (Tf / T2) + n2 R ln ((V1 + V2) /V2)
Then I don't know how to preceed with the volume term...
Clara Chung
- 300
- 13
Sorry I have another question... Is entropy an unique property? Is there two different equilibrium states with different values of entropy? For example... Energy is a state function... but it is ok for two different equilibrium states to have the same temperature (but with different volume/ pressure) etc...
- 23,704
- 5,917
Very nice job, but the problem is a little over-specified and there should be n's in front of the Cv's. You need to use the ideal gas law for the initial conditions to express V1 and V2 in terms of P1, T1, n1 and P2, T2, n2. Also, if there is a sliding barrier present between the two gases, the final volume of each gas is not equal to the total volume of the container. For one of the gases, its volume increases, and, for the other gas, its volume decreases by an equal amount. The change in volume of each is determined by requiring that the final pressure is the same for each. So, with these comments, please go back and determine the final pressure.Clara Chung said:If I use solve it as an ideal gas, let the system with T1 , P1, V1 and n1 be system 1.
Let the system with T2 , P2, V2 and n2 be system 2.
Let the final temperature be Tf and final pressure be Pf.
I try to find the change of entropy between two equilibrium states, so if change in entropy = 0, the two equilibrium states will be equivalent?(I am not sure about this statement.
The internal energy of the system 1 and system 2 remains the same, i.e. n1 T1 + n2 T2 = (n1 + n2 )Tf.
Consider system 1,
the first reversible step will be an isothermal expansion from V1 to V1 + V2,
the entropy change in this reversible process is n1 R ln (V1 + V2 / V1).
the second step will be an isochoric heating to slowly increase the temperature from T1 to Tf,
dQrev = Cv dT
ΔS1=Cv ln (Tf / T1) + n1 R ln (V1 + V2 /V1)
Similarly for system 2,
so the total change in entropy is:
ΔS1 + ΔS2
= Cv ln (Tf / T1) + n1 R ln ((V1 + V2) /V1) + Cv ln (Tf / T2) + n2 R ln ((V1 + V2) /V2)
Then I don't know how to preceed with the volume term...
- 23,704
- 5,917
Yes. It is a physical property of the material.Clara Chung said:Sorry I have another question... Is entropy an unique property?
Sure. You already showed that for an ideal gas, where the entropy is a function of temperature and specific volume (or, equivalently, temperature and pressure)Is there two different equilibrium states with different values of entropy? For example... Energy is a state function... but it is ok for two different equilibrium states to have the same temperature (but with different volume/ pressure) etc...
Clara Chung
- 300
- 13
Chestermiller said:Very nice job, but the problem is a little over-specified and there should be n's in front of the Cv's. You need to use the ideal gas law for the initial conditions to express V1 and V2 in terms of P1, T1, n1 and P2, T2, n2. Also, if there is a sliding barrier present between the two gases, the final volume of each gas is not equal to the total volume of the container. For one of the gases, its volume increases, and, for the other gas, its volume decreases by an equal amount. The change in volume of each is determined by requiring that the final pressure is the same for each. So, with these comments, please go back and determine the final pressure.
P1 V1 = n1 R T1
P2 V2 = n2 R T2
Pf (V1+V2) = (n1 + n2) R Tf
so Pf = (n1 + n2) R Tf / (V1+V2)
The new volume of system 1 will be
V1f= n1 R Tf / [(n1 + n2) R Tf / (V1+V2)]
= (V1+V2) n1 / (n1+n2)
Similarly for system 2 the change in volume will be
V2f= n2 R Tf / [(n1 + n2) R Tf / (V1+V2)]
= (V1+V2) n2 / (n1+n2)
It is quite surprising that the volume each gas occupied is not the same as the total volume...
Should I proceed in the calculation of change in entropy with these results? However, the calculation will be so complicated because n1 and n2 will contribute as power in the logarithm...?
Clara Chung
- 300
- 13
So, if I have 2 systems, each with the same value of entropy, however the temperature of system A is higher than the temperature in system B, will there be a heat transfer from system A to system B?Chestermiller said:Yes. It is a physical property of the material.
Sure. You already showed that for an ideal gas, where the entropy is a function of temperature and specific volume (or, equivalently, temperature and pressure)
Mathematically, entropy is a function of T and V, i.e. S(T,V), is it possible that
S(T1 , V1) = S(T2, V2) for different temperature and volume? (This would imply two system are not in equilibrium even if ΔS=0 between the two systems)
or
S depends only on temperature?
- 23,704
- 5,917
Eliminate V1 and V2 using the initial conditions to express Pf exclusively in terms of T1, T2, P1, P2, n1, and n2. Then calculate entropy change of each gas from $$\Delta S=nC_p\Delta (\ln{T})-nR\Delta (\ln{P})$$
- 23,704
- 5,917
Sure.Clara Chung said:So, if I have 2 systems, each with the same value of entropy, however the temperature of system A is higher than the temperature in system B, will there be a heat transfer from system A to system B?
Let's see how it plays out when we do what I just said in my previous post.Mathematically, entropy is a function of T and V, i.e. S(T,V), is it possible that
S(T1 , V1) = S(T2, V2) for different temperature and volume? (This would imply two system are not in equilibrium even if ΔS=0 between the two systems)
or
S depends only on temperature?
- 23,704
- 5,917
To simplify things (temporarily), let's just, for now, focus on the case where n1=n2=1
- 23,704
- 5,917
What happened to the n2 chamber?Clara Chung said:
Clara Chung
- 300
- 13
If my equation is correct, the total change in entropy for n1=n2=1 will beChestermiller said:What happened to the n2 chamber?
??
Attachments
- 23,704
- 5,917
If I multiply and divide the expression in brackets of the 2nd term in your relationship by ##T_1T_2##, I obtain:Clara Chung said:If my equation is correct, the total change in entropy for n1=n2=1 will be
View attachment 236705
??
$$\Delta S=C_p\ln{\left(\frac{(T_1+T_2)^2}{T_1T_2}\right)}-R\ln{\left(\frac{(T_1+T_2)^2}{T_1T_2}\frac{\left(\frac{T_1}{P_1}\right)\left(\frac{T_2}{P_2}\right)}{\left(\frac{T_1}{P_1}+\frac{T_2}{P_2}\right)^2}\right)}$$This can be rewritten as:
$$\Delta S=C_v\ln{\left(\frac{(T_1+T_2)^2}{T_1T_2}\right)}+R\ln{\left(\frac{\left(\frac{T_1}{P_1}+\frac{T_2}{P_2}\right)^2}{\left(\frac{T_1}{P_1}\right)\left(\frac{T_2}{P_2}\right)}\right)}$$
OK so far? Note the similarity in mathematical form for the two logarithmic terms. I will continue after you indicate you are comfortable with these results.
Clara Chung
- 300
- 13
OK. I am comfortable with the resultChestermiller said:If I multiply and divide the expression in brackets of the 2nd term in your relationship by ##T_1T_2##, I obtain:
$$\Delta S=C_p\ln{\left(\frac{(T_1+T_2)^2}{T_1T_2}\right)}-R\ln{\left(\frac{(T_1+T_2)^2}{T_1T_2}\frac{\left(\frac{T_1}{P_1}\right)\left(\frac{T_2}{P_2}\right)}{\left(\frac{T_1}{P_1}+\frac{T_2}{P_2}\right)^2}\right)}$$This can be rewritten as:
$$\Delta S=C_v\ln{\left(\frac{(T_1+T_2)^2}{T_1T_2}\right)}+R\ln{\left(\frac{\left(\frac{T_1}{P_1}+\frac{T_2}{P_2}\right)^2}{\left(\frac{T_1}{P_1}\right)\left(\frac{T_2}{P_2}\right)}\right)}$$
OK so far? Note the similarity in mathematical form for the two logarithmic terms. I will continue after you indicate you are comfortable with these results.
- 23,704
- 5,917
We are missing a factor of 4 in the denominator of each of the logarithmic terms. For example, for the first term, the final temperature is ##(T_1+T_2)/2##
I can write $$T_1T_2=\left(\frac{(T_1+T_2)}{2}\right)^2-\left(\frac{(T_1-T_2)}{2}\right)^2$$ so $$\frac{(T_1+T_2)^2}{4T_1T_2}=\frac{1}{\left[1-\frac{1}{4}\left(\frac{\Delta T}{\bar{T}}\right)^2\right]}$$ where ##\bar{T}=\frac{(T_1+T_2)}{2}##and ##\Delta T=(T_1-T_2)##. This expression is >1 for all values ##\Delta T\neq 0##, which means it always makes a positive contribution to the entropy change.
What does the same procedure tell you for the second term in the entropy change equation.
I can write $$T_1T_2=\left(\frac{(T_1+T_2)}{2}\right)^2-\left(\frac{(T_1-T_2)}{2}\right)^2$$ so $$\frac{(T_1+T_2)^2}{4T_1T_2}=\frac{1}{\left[1-\frac{1}{4}\left(\frac{\Delta T}{\bar{T}}\right)^2\right]}$$ where ##\bar{T}=\frac{(T_1+T_2)}{2}##and ##\Delta T=(T_1-T_2)##. This expression is >1 for all values ##\Delta T\neq 0##, which means it always makes a positive contribution to the entropy change.
What does the same procedure tell you for the second term in the entropy change equation.
Clara Chung
- 300
- 13
Chestermiller said:We are missing a factor of 4 in the denominator of each of the logarithmic terms. For example, for the first term, the final temperature is ##(T_1+T_2)/2##
I can write $$T_1T_2=\left(\frac{(T_1+T_2)}{2}\right)^2-\left(\frac{(T_1-T_2)}{2}\right)^2$$ so $$\frac{(T_1+T_2)^2}{4T_1T_2}=\frac{1}{\left[1-\frac{1}{4}\left(\frac{\Delta T}{\bar{T}}\right)^2\right]}$$ where ##\bar{T}=\frac{(T_1+T_2)}{2}##and ##\Delta T=(T_1-T_2)##. This expression is >1 for all values ##\Delta T\neq 0##, which means it always makes a positive contribution to the entropy change.
What does the same procedure tell you for the second term in the entropy change equation.
And the same expression with T bar and ΔT replaced by X bar and ΔX. 2 X bar is always larger than ΔX, so the contribution to entropy is always positive. The change in entropy is zero when ΔT=0 and ΔX=0
Attachments
Clara Chung
- 300
- 13
Therefore the entropy change is zero if and if only the temperature and pressure are equal between two equilibrium states. Does it mean if two states have the same value of entropy, the two states are identical? (States with different temperature but the same entropy don't exist?)
- 23,704
- 5,917
No. It only means that, at equilibrium, entropy is a minimum with respect to changes in the intensive variables.Clara Chung said:Therefore the entropy change is zero if and if only the temperature and pressure are equal between two equilibrium states. Does it mean if two states have the same value of entropy, the two states are identical? (States with different temperature but the same entropy don't exist?)
Similar threads
- Replies
- 23
- Views
- 4K
Engineering
How do I model a Thorium Reactor thermal system?
- Replies
- 7
- Views
- 1K
- Replies
- 1
- Views
- 1K
- Replies
- 3
- Views
- 2K
- Replies
- 8
- Views
- 2K
- Replies
- 8
- Views
- 2K
- Replies
- 9
- Views
- 3K
- Replies
- 10
- Views
- 1K
- Replies
- 16
- Views
- 2K
- Replies
- 1
- Views
- 2K
Hot Threads
-
Engineering Why is my output current so low in this Transconductance Amplifier cell?
- Started by arhzz
- Replies: 13
- Engineering and Comp Sci Homework Help
-
LTspice: Implementing a Single Balanced BJT Mixer
- Started by SumDood_
- Replies: 17
- Engineering and Comp Sci Homework Help
-
Max water pressure allowable on solar panels
- Started by ethanesh
- Replies: 16
- Engineering and Comp Sci Homework Help
-
Spiral scissor lift statics
- Started by carlcla
- Replies: 4
- Engineering and Comp Sci Homework Help
Recent Insights
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 7
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 0
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 1
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 91
- General Math
-
Insights Why Vector Spaces Explain The World: A Historical Perspective
- Started by fresh_42
- Replies: 0
- General Math