b_andries
- 13
- 0
Hi guys,
Can someone explain me please how to find the following question?
Which diagram represents the behavior of an ideal gas the most?
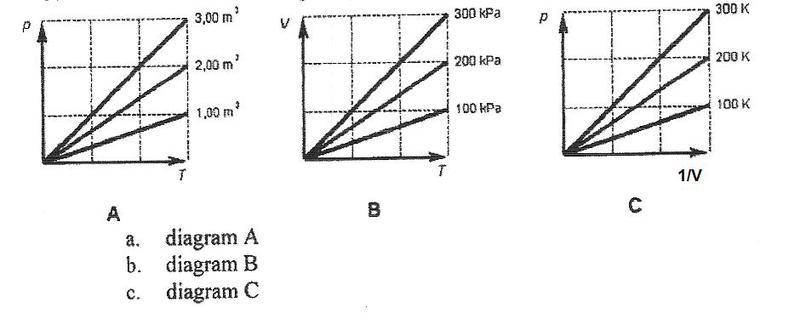
I know it's C but I don't know why. They all seem to represent the behavior of a ideal gas.
But why C mor than the others?
Thanks
Can someone explain me please how to find the following question?
Which diagram represents the behavior of an ideal gas the most?
I know it's C but I don't know why. They all seem to represent the behavior of a ideal gas.
But why C mor than the others?
Thanks