- #1
samven582
- 1
- 0
I need help identifying this compound. I already know at 3000cm-1 and less than 3500cm-1 its a C -H. Less then 3000cm-1 must be of C-H alkane. Thats pretty much all I have identify. What I really need help is identifying the rest andnaming the compound. Any help would be appericated.
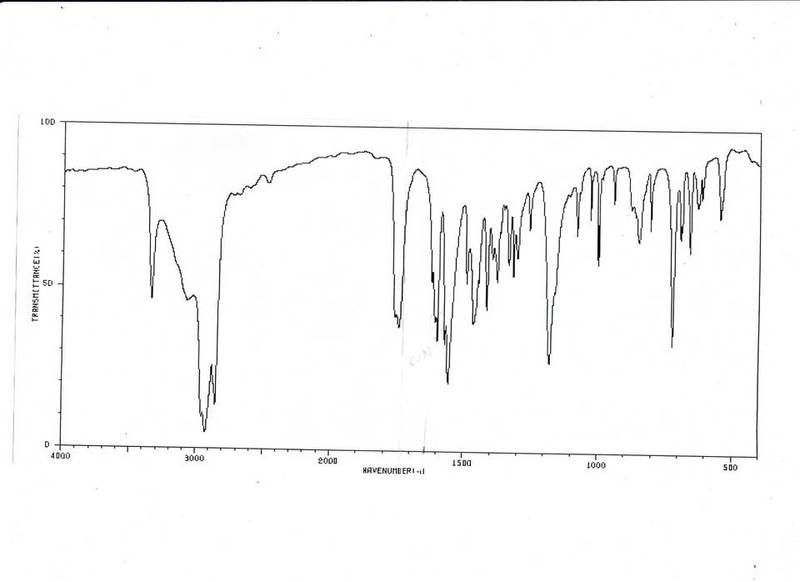
 Gema
Gema