jmex
- 59
- 3
Hello,
From the figure how is it possible to say that this line shows amount of liquid while other is amount of solid. In the figure they showed MP is amount of liquid while OM is amount of solid. How?
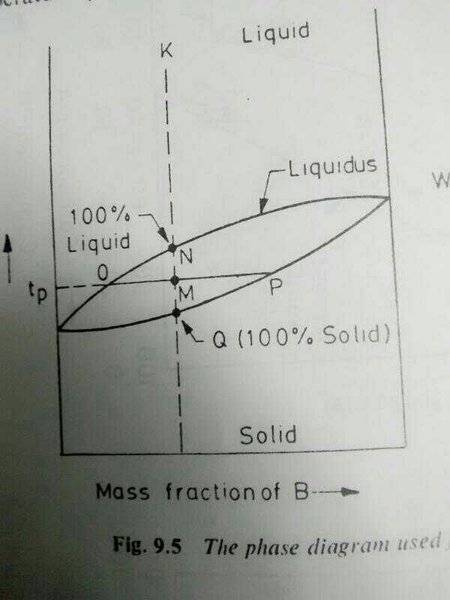
From the figure how is it possible to say that this line shows amount of liquid while other is amount of solid. In the figure they showed MP is amount of liquid while OM is amount of solid. How?