oksuz_
- 70
- 3
Hi,
For an ideal Rankine cycle,
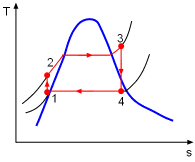
the temperature-specific entropy diagram can be given as follows,
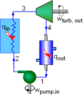
The pump in this cycle is isentropically 100% efficient.
My question is that why the temperature of the water increases as it flows through the pump (point 1 to 2) if the pump is 100% efficient. I can understand why point 2 is not on the saturation line (need higher temperature at a higher pressure to make a liquid saturated).
Thank you in advance.
For an ideal Rankine cycle,
the temperature-specific entropy diagram can be given as follows,
The pump in this cycle is isentropically 100% efficient.
My question is that why the temperature of the water increases as it flows through the pump (point 1 to 2) if the pump is 100% efficient. I can understand why point 2 is not on the saturation line (need higher temperature at a higher pressure to make a liquid saturated).
Thank you in advance.
Attachments
Last edited by a moderator: