Zouatine
- 19
- 0
Member warned that some effort must be shown
- Homework Statement
- thermodynamic
- Relevant Equations
- I think the answer is b , is that true ?
Hi every one , I have this question today :
The two arrows in the figure show two thermodynamic processes of a certain amount of ideal gas. The dashed line is a hyperbola. Which of the following statements is not true?
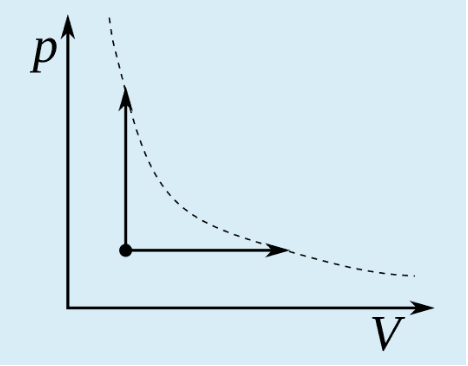
a. The heat transfer is the same in the two processes.
b. The temperature change is the same in the two processes.
c. The change in internal energy is the same in the two processes.
The two arrows in the figure show two thermodynamic processes of a certain amount of ideal gas. The dashed line is a hyperbola. Which of the following statements is not true?
a. The heat transfer is the same in the two processes.
b. The temperature change is the same in the two processes.
c. The change in internal energy is the same in the two processes.
