- #1
ValeForce46
- 40
- 3
I'll put pictures from the book as I think they are relevant to understand the problem:
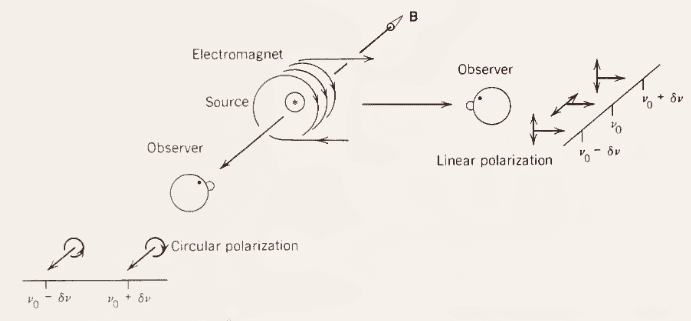
I have trouble understanding the case where the observer watches the source in a direction perpendicular to the magnetic field. The electron will rotate around B axis, so the observer will only see a linear oscillation of the electron hence linearly polarized light.
But how can the Lorentz force explain the splitting of spectral lines? The book suggest to view the linear oscillation as a combination of two counter-rotating motions like this:
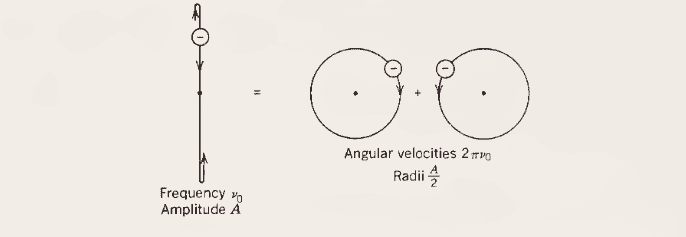
But if this is the case, the Lorentz force would act in a plane perpendicular to the image so it won't explain the change of the frequency of the circular motion of the electron (and so the Zeeman splitting, classically).
Instead the situation is clear when we observe along the direction of B, as in that case Lorentz force would act radially.
I have trouble understanding the case where the observer watches the source in a direction perpendicular to the magnetic field. The electron will rotate around B axis, so the observer will only see a linear oscillation of the electron hence linearly polarized light.
But how can the Lorentz force explain the splitting of spectral lines? The book suggest to view the linear oscillation as a combination of two counter-rotating motions like this:
But if this is the case, the Lorentz force would act in a plane perpendicular to the image so it won't explain the change of the frequency of the circular motion of the electron (and so the Zeeman splitting, classically).
Instead the situation is clear when we observe along the direction of B, as in that case Lorentz force would act radially.