You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Specific Heat Capacity: Comparing Spheres
- Thread starter fabrc
- Start date
AI Thread Summary
The discussion clarifies the distinction between "specific heat" and "heat capacity," emphasizing that specific heat is a property of matter while heat capacity is an object's attribute based on its specific heat and mass. It suggests that understanding these concepts is crucial for determining which sphere has a higher specific heat capacity. A link to a Wikipedia page is provided, reinforcing that specific heat capacity is defined as heat capacity per unit mass. The conversation hints at a deeper inquiry regarding the spheres beyond just their specific heat capacities. Overall, grasping these definitions is essential for conducting relevant experiments.
Science news on Phys.org
NascentOxygen
Staff Emeritus
Science Advisor
Homework Helper
- 9,248
- 1,080
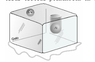
Hi fabrc. Can you explain what the photo is showing us?
gsal
- 1,065
- 54
First, I am afraid you are gluing the words of two different concepts...probably because they are directly related, but you need to keep in mind that they are two separate concepts.
To be sure, there is no "Specific Heat Capacity"; instead, you have two concepts:
"Specific Heat", this is a property of matter.
"Heat Capacity", this is an attribute on an object that depends on its "specific heat" and on its mass.
Once you understand these two concepts and how they relate to each other (go back to read the textbook or google...I got a few links right away), you should be able to explain what experiment you can do to answer the correct question.
To be sure, there is no "Specific Heat Capacity"; instead, you have two concepts:
"Specific Heat", this is a property of matter.
"Heat Capacity", this is an attribute on an object that depends on its "specific heat" and on its mass.
Once you understand these two concepts and how they relate to each other (go back to read the textbook or google...I got a few links right away), you should be able to explain what experiment you can do to answer the correct question.
rtsswmdktbmhw
- 38
- 2
I googled it, as you suggested. This is the first link: http://en.wikipedia.org/wiki/Heat_capacitygsal said:First, I am afraid you are gluing the words of two different concepts...probably because they are directly related, but you need to keep in mind that they are two separate concepts.
To be sure, there is no "Specific Heat Capacity"; instead, you have two concepts:
"Specific Heat", this is a property of matter.
"Heat Capacity", this is an attribute on an object that depends on its "specific heat" and on its mass.
Once you understand these two concepts and how they relate to each other (go back to read the textbook or google...I got a few links right away), you should be able to explain what experiment you can do to answer the correct question.
Taken from the second paragraph on that page: '. . . and the specific heat capacity, often simply called specific heat, is the heat capacity per unit mass of a material.'
CWatters
Science Advisor
Homework Helper
Gold Member
- 10,544
- 2,324
If you take the question at face value... The specific heat capacity depends only on the material the sphere is made of and that's stated on the image.
However I suspect there is more to the question.
However I suspect there is more to the question.
I need to calculate the amount of water condensed from a DX cooling coil per hour given the size of the expansion coil (the total condensing surface area), the incoming air temperature, the amount of air flow from the fan, the BTU capacity of the compressor and the incoming air humidity. There are lots of condenser calculators around but they all need the air flow and incoming and outgoing humidity and then give a total volume of condensed water but I need more than that. The size of the...
Let's say we have a cylinder of volume V1 with a frictionless movable piston and some gas trapped inside with pressure P1 and temperature T1. On top of the piston lay some small pebbles that add weight and essentially create the pressure P1. Also the system is inside a reservoir of water that keeps its temperature constant at T1.
The system is in equilibrium at V1, P1, T1.
Now let's say i put another very small pebble on top of the piston (0,00001kg)
and after some seconds the system...
Similar threads
- Replies
- 2
- Views
- 3K
- Replies
- 8
- Views
- 2K
- Replies
- 28
- Views
- 3K
- Replies
- 2
- Views
- 2K
- Replies
- 4
- Views
- 3K
- Replies
- 1
- Views
- 3K
- Replies
- 22
- Views
- 3K
- Replies
- 4
- Views
- 1K
- Replies
- 2
- Views
- 2K
- Replies
- 9
- Views
- 3K
Hot Threads
-
I Explain Bernoulli at the molecular level?
- Started by user079622
- Replies: 251
- Classical Physics
-
B Simple mass/scale puzzle
- Started by DaveC426913
- Replies: 206
- Classical Physics
-
I How deep does it need to go for a physics theory to be consistent?
- Started by Aleberto69
- Replies: 50
- Classical Physics
-
I Solving a momentum problem where masses change after the collision
- Started by rdemyan
- Replies: 35
- Classical Physics
-
B Torque Calculation - Not a basic application
- Started by TeeBeeCee
- Replies: 37
- Classical Physics
Recent Insights
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 7
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 0
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 0
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 78
- General Math
-
Insights Why Vector Spaces Explain The World: A Historical Perspective
- Started by fresh_42
- Replies: 0
- General Math