ReuvenD10
- 8
- 1
- Homework Statement
- Get values of thermal expansion coefficient
- Relevant Equations
- below
Hello everyone,
Once I got through the VDW state equation I came to the expression of the thermal expansion coefficient. When I place the values I get an illogical answer. Is there a problem with the units? (Please ignore the values)
Thanks.
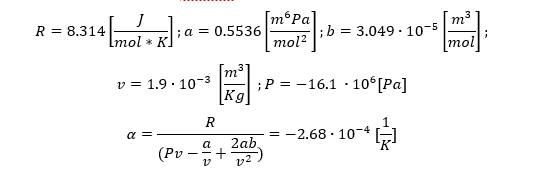
This is the unit equation I get to and get stuck:
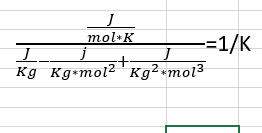
Once I got through the VDW state equation I came to the expression of the thermal expansion coefficient. When I place the values I get an illogical answer. Is there a problem with the units? (Please ignore the values)
Thanks.
This is the unit equation I get to and get stuck:
Attachments
Last edited by a moderator: