- #1
forty
- 135
- 0
Consider the cycle shown below for n moles of an ideal gas. The gas has a heat capacity of Cv=(3/2)nR and is initially at a temperature T0, a pressure P0 and a volume V0. The process a->b consists of a quasistatic, isobaric expansion to twice the initial volume. The process b->c consists of a quasistatic, isochoric decrease in pressure and the process c->a is a quasistatic , adiabatic compression.
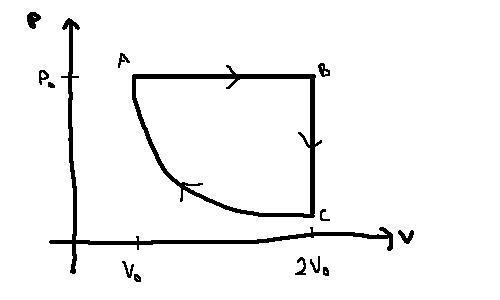
(a) In terms of the initial pressure at point a, P0, what is the pressure at the point c?
Worked this out to be Pc = 2-ɣP0
(b) In terms of the initial temperature at point a, T0, what is the temperature at points b and c.
Worked these out to be Tb = 2T0 and Tc = 4-1/fT0
(c) Construct a table which displays for each of the three processes in this cycle:
The work done, the heat transfer, the change in internal energy, the change in enthalpy and the change in entropy. Express your answers in terms of R, the universal gas constant, n, the number of moles of the gas present and T0, the initial temperature at point a.
For a->b:
Work done: W=-PΔV=-P0(2V0-V0)=-P0V0=nRT0
Heat transfer: 0?
Change in internal energy: (f/2)nRΔT=(f/2)nR(Tb-Ta)=(f/2)nR(2T0-T0)
Change in enthalpy: ΔH=ΔU+PΔV=(f/2)nR(4-1/fT0-2T0)+nRT0
Change in entropy: ΔS=nRln(Vf/Vi)=nRln(2)
For b->c:
Work done: 0
Heat transfer: Does this = ΔU?
Change in internal energy: ΔU=(f/2)nRΔT=(f/2)nR(Tc-Tb)=(f/2)nR(4-1/fT0-2T0)
Change in enthalpy: ΔH=ΔU+ΔPV=(f/2)nR(4-1/fT0-2T0)+0
Change in entropy: ΔS=nRln(Vf/Vi) Does this work here?
I'm just not sure if I'm on the right track... Especially for part (c). Any help with this would be appreciated.
(a) In terms of the initial pressure at point a, P0, what is the pressure at the point c?
Worked this out to be Pc = 2-ɣP0
(b) In terms of the initial temperature at point a, T0, what is the temperature at points b and c.
Worked these out to be Tb = 2T0 and Tc = 4-1/fT0
(c) Construct a table which displays for each of the three processes in this cycle:
The work done, the heat transfer, the change in internal energy, the change in enthalpy and the change in entropy. Express your answers in terms of R, the universal gas constant, n, the number of moles of the gas present and T0, the initial temperature at point a.
For a->b:
Work done: W=-PΔV=-P0(2V0-V0)=-P0V0=nRT0
Heat transfer: 0?
Change in internal energy: (f/2)nRΔT=(f/2)nR(Tb-Ta)=(f/2)nR(2T0-T0)
Change in enthalpy: ΔH=ΔU+PΔV=(f/2)nR(4-1/fT0-2T0)+nRT0
Change in entropy: ΔS=nRln(Vf/Vi)=nRln(2)
For b->c:
Work done: 0
Heat transfer: Does this = ΔU?
Change in internal energy: ΔU=(f/2)nRΔT=(f/2)nR(Tc-Tb)=(f/2)nR(4-1/fT0-2T0)
Change in enthalpy: ΔH=ΔU+ΔPV=(f/2)nR(4-1/fT0-2T0)+0
Change in entropy: ΔS=nRln(Vf/Vi) Does this work here?
I'm just not sure if I'm on the right track... Especially for part (c). Any help with this would be appreciated.
