- #1
WCMU101
- 14
- 0
Hey all. I'm going through my textbook at the moment and struggling to figure out something.
Here is the ideal Brayton cycle (same as in my text).
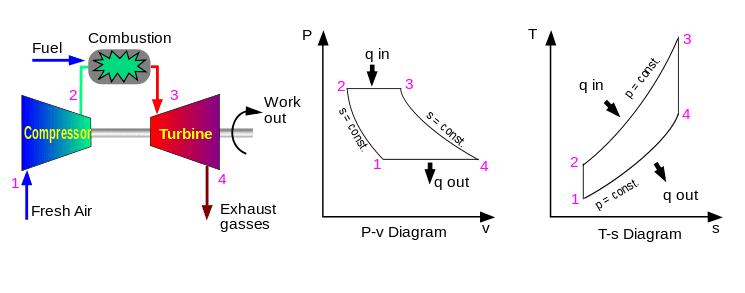
Now I want to find q in, so:
The first law of thermodynamics can be written as (neglecting changes in ke and pe):
h_in - h_out + q_in - q_out + w_in - w_out = change in energy.
Focusing on the combustion process - 2-3:
h2 - h3 + q_in = change in energy
As we are assuming the flow is steady, the change in energy must be zero, thus:
q_in = h3 - h2
For a thermally perfect gas h depends only on T (h = c_p * T), So:
q_in = c_p(T3 - T2)
Now most sites I've looked at get this result. However in my textbook (a textbook for propulsion), the temperatures are stated at total temperatures, so the textbook says:
q_in = c_p(T_t3 - T_t2)
However for q_out the text states the same formula as most other stuff I've seen:
q_out = c_p(T4 - T1)
Just the static temperatures.
I'm really lost. Using the total temperatures is important because it allows us to get the Mach number in the final thermal efficiency formula.
Any help would be greatly appreciated.
Nick.
Here is the ideal Brayton cycle (same as in my text).
Now I want to find q in, so:
The first law of thermodynamics can be written as (neglecting changes in ke and pe):
h_in - h_out + q_in - q_out + w_in - w_out = change in energy.
Focusing on the combustion process - 2-3:
h2 - h3 + q_in = change in energy
As we are assuming the flow is steady, the change in energy must be zero, thus:
q_in = h3 - h2
For a thermally perfect gas h depends only on T (h = c_p * T), So:
q_in = c_p(T3 - T2)
Now most sites I've looked at get this result. However in my textbook (a textbook for propulsion), the temperatures are stated at total temperatures, so the textbook says:
q_in = c_p(T_t3 - T_t2)
However for q_out the text states the same formula as most other stuff I've seen:
q_out = c_p(T4 - T1)
Just the static temperatures.
I'm really lost. Using the total temperatures is important because it allows us to get the Mach number in the final thermal efficiency formula.
Any help would be greatly appreciated.
Nick.