Jaydude
- 3
- 0
I'm stuck on a question, part d below:
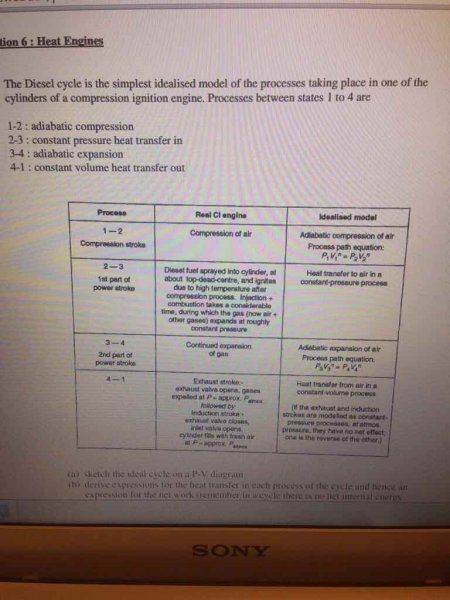
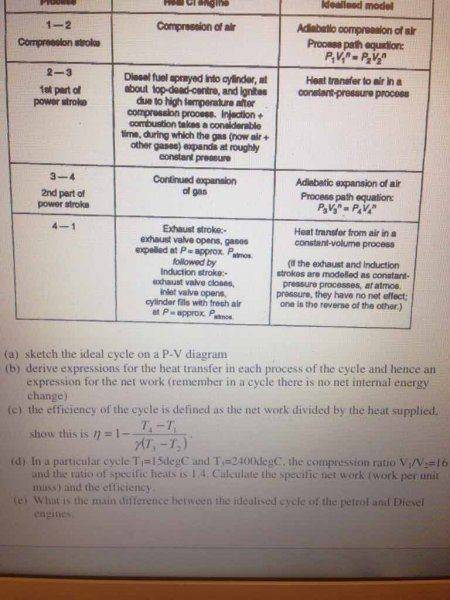
My attempt:
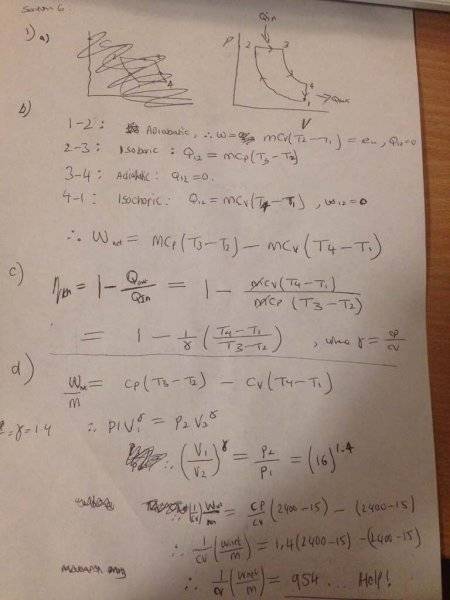
I reckon that T4 = T3 and T2 = T1, otherwise I don't know how else you can get T4 and T2...?
Once I got first part of d, I reckon I could do efficiency using part c that I derived.
I don't see where I'm supposed to use compression ratios in this question, if someone can direct me to the right path please!
(If this is supposed to be in advanced physics section, please let me know for next time!)
Kind regards,
Jay
My attempt:
I reckon that T4 = T3 and T2 = T1, otherwise I don't know how else you can get T4 and T2...?
Once I got first part of d, I reckon I could do efficiency using part c that I derived.
I don't see where I'm supposed to use compression ratios in this question, if someone can direct me to the right path please!
(If this is supposed to be in advanced physics section, please let me know for next time!)
Kind regards,
Jay