Jeremy Stone
Hey Guys!
I need some help for solving question 2 of this example as my HSC exam is in 5 days and I want to be able to solve this question if I receive it on the paper.
Question:
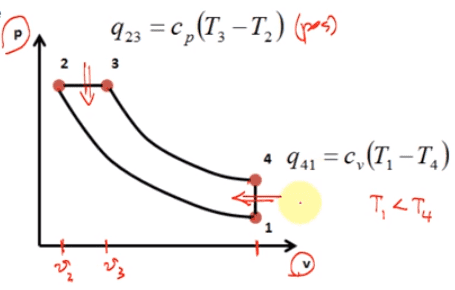
Graph:
Answer for Q1 (Just the formula) :
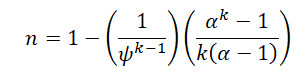
Work I have done towards this question:
I have realized that from 1-2, there is an increase in pressure, decrease in volume and increase in temperature I have realized that from 4-1, there is an decrease in pressure, constant volume and decrease in temperature I have realized that from 3-4, there is an decrease in pressure, increase in volume and decrease in temperature I am not sure where to go from here and what values to substitue in
I need some help for solving question 2 of this example as my HSC exam is in 5 days and I want to be able to solve this question if I receive it on the paper.
Question:
Graph:
Answer for Q1 (Just the formula) :
Work I have done towards this question:
I have realized that from 1-2, there is an increase in pressure, decrease in volume and increase in temperature I have realized that from 4-1, there is an decrease in pressure, constant volume and decrease in temperature I have realized that from 3-4, there is an decrease in pressure, increase in volume and decrease in temperature I am not sure where to go from here and what values to substitue in

