- #1
vijayantv
- 23
- 1
- TL;DR Summary
- questions about Black body radiation
I have questions about Black body radiation. see the attached image
1). It explains that the Spectral radiance measurement of 7000K temperature is the same as both 300 and 520 nm wavelength light.
See here both A and B shows 60.
Is my understanding correct?
2). Is spectral radiance the number of photons? If not, what exactly it is?
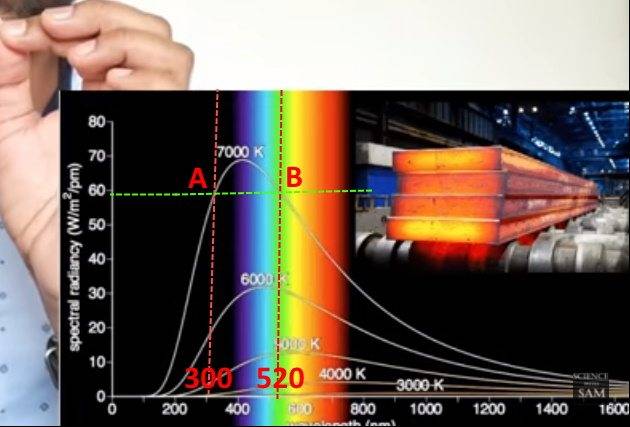
Please clarify.
1). It explains that the Spectral radiance measurement of 7000K temperature is the same as both 300 and 520 nm wavelength light.
See here both A and B shows 60.
Is my understanding correct?
2). Is spectral radiance the number of photons? If not, what exactly it is?
Please clarify.