getcarter
- 35
- 0
Verify that the uncertainty principle can be expressed in the form
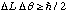 , where
, where
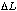 is the uncertainty in the angular momentum of a particle, and
is the uncertainty in the angular momentum of a particle, and
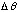 is the uncertainty in its angular position. (You may think of a particle, mass m, moving in a circle of fixed radius r, with speed v)
is the uncertainty in its angular position. (You may think of a particle, mass m, moving in a circle of fixed radius r, with speed v)
b) At what uncertainty in L will the angular position of a particle become completely indeterminate?
b) At what uncertainty in L will the angular position of a particle become completely indeterminate?
Last edited by a moderator: