- #1
yinnxz
- 3
- 0
- Homework Statement
- Imagine that it were possible to construct
a reservoir at −5 K (below absolute zero).
Suppose you ran an engine and used the −5
K reservoir as the cold reservoir.
Would such an engine violate the second
law of thermodynamics?
1. Yes; the engine efficiency would be greater
than 100 %.
2. No, the engine efficiency would be high,
but reasonable.
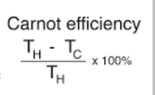
- Relevant Equations
- η = Energy out/Total Energy * 100
I don't understand, can you calculate efficiency only using the temperature?
 ##\qquad## !
##\qquad## !