- #1
kma
- 27
- 0
1
In recent days I have done a few experiments measuring the current of water as it goes up from 9 volts up to 36 volts, and following Ohms law to convert it to resistance. And I discovered a very interesting trend. In between 9 and 18 volts, there is a massive drop in resistance (by around a 40% reduction) but then as I go up to 27 volts, its a 5% reduction, and is even less of a reduction when reaching 36 volts. I've done this experiment a few times and this has continued to happen. This is it visualised on a graph;

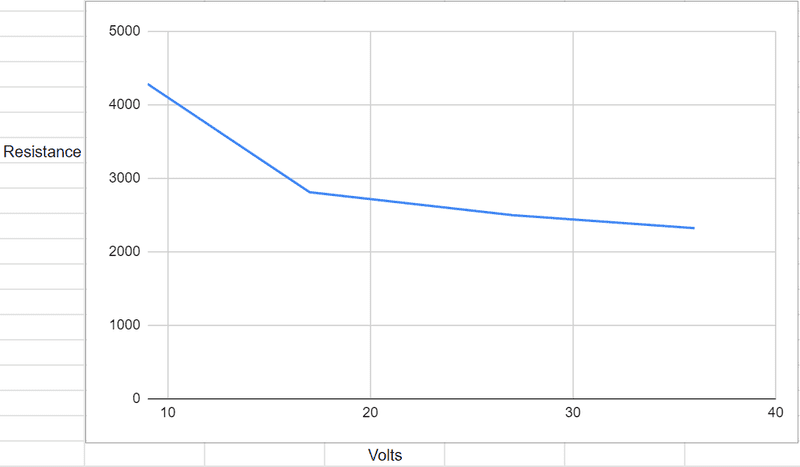
I am curious to know why this happens, why there seems to be a reduction as I go from 9 to 18 volts, yet the reduction seems to reduce at 27 volts and reduce further at 36. Is there a reason that? And as I go further up the voltages (don't want to test with higher), does this continue with the reduction in resistance continuously reducing, and if not at what voltage does it change?
Specifically I kinda want to know what resistance can I expect at around 240 volts
In recent days I have done a few experiments measuring the current of water as it goes up from 9 volts up to 36 volts, and following Ohms law to convert it to resistance. And I discovered a very interesting trend. In between 9 and 18 volts, there is a massive drop in resistance (by around a 40% reduction) but then as I go up to 27 volts, its a 5% reduction, and is even less of a reduction when reaching 36 volts. I've done this experiment a few times and this has continued to happen. This is it visualised on a graph;
I am curious to know why this happens, why there seems to be a reduction as I go from 9 to 18 volts, yet the reduction seems to reduce at 27 volts and reduce further at 36. Is there a reason that? And as I go further up the voltages (don't want to test with higher), does this continue with the reduction in resistance continuously reducing, and if not at what voltage does it change?
Specifically I kinda want to know what resistance can I expect at around 240 volts