You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
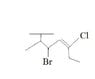
What Is the Correct Chemical Structure for Hexane?
- Thread starter ahazen
- Start date
-
- Tags
- Structure
AI Thread Summary
The discussion centers on identifying the correct chemical structure for hexane. Participants express confusion over labeling, with suggestions including 2-methyl butane and 3-bromo-4,5-ene. There is uncertainty about the meaning of "T," with one participant speculating it could indicate isopropyl. The conversation also considers the possibility that the structure may not be hexane but rather an alkene or octane. Ultimately, clarity on naming conventions and structural identification remains a key focus.
Physics news on Phys.org
Borek
Mentor
- 29,132
- 4,556
It is not a hexane.
Does the "T" mean isopropyl?
ahazen
- 49
- 0
I'm not sure what the "T" is. I can't find the structure in my book:( If its not a hexane, then it has to be an octane.
chemisttree
Science Advisor
Homework Helper
Gold Member
- 3,950
- 781
It looks like an alkene that is anchored to a solid support (the "T") or the side chain for a polymer. If that is the case, you name it but it ends with "-yl".
ahazen
- 49
- 0
ok, thank you:)
I don't get how to argue it. i can prove: evolution is the ability to adapt, whether it's progression or regression from some point of view, so if evolution is not constant then animal generations couldn`t stay alive for a big amount of time because when climate is changing this generations die. but they dont. so evolution is constant.
but its not an argument, right?
how to fing arguments when i only prove it.. analytically, i guess it called that (this is indirectly related to biology, im...
Similar threads
- Replies
- 2
- Views
- 2K
- Replies
- 2
- Views
- 2K
- Replies
- 2
- Views
- 3K
- Replies
- 1
- Views
- 2K
- Replies
- 4
- Views
- 2K
- Replies
- 4
- Views
- 11K
- Replies
- 1
- Views
- 3K
- Replies
- 1
- Views
- 2K
- Replies
- 1
- Views
- 3K
- Replies
- 3
- Views
- 3K
Hot Threads
-
Confusion regarding a chemical kinetics problem
- Started by palaphys
- Replies: 12
- Biology and Chemistry Homework Help
-
Biology Prove that evolution is constant (Provide at least two arguments to support your position)
- Started by yo yo
- Replies: 27
- Biology and Chemistry Homework Help
Recent Insights
-
Insights Why Entangled Photon-Polarization Qubits Violate Bell’s Inequality
- Started by Greg Bernhardt
- Replies: 26
- Quantum Interpretations and Foundations
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 11
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 5
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 1
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 105
- General Math