TFM
- 1,016
- 0
Homework Statement
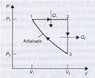
A hypothetical engine, with an ideal gas as the working substance, operates on the cycle shown in Figure 1. Show that the efficiency of this engine is
e = 1 - \frac{1}{\gamma}\left(\frac{1 - \frac{p_3}{p_1}}{1 - \frac{v_3}{v_1}}\right)
Where \gamma = \frac{c_p}{c_v}
Homework Equations
Efficiency = Benefit/Cost
Benefit = Work out
Cost = Heat in
PV = nRt
\Delta U = Q_{hot} - Q_{cold} - Work
Work = pdv
The Attempt at a Solution
The Graph is attached, but basically it has a Adiabatic curve, which at the bottom goes up vertically, then left horizontally, back to make a cycle.
So far I have:
E = Work/Cost
Work = pdv
Cost = Q
w = pdv
Since p is not constant, use ideal law,
w = \frac{nRt}{v}dv
thus
w = \frac{nRt}{v}dv
w = nRt [ln(v)]^{v_1}_{v_2}
I also have:
e = 1 - \frac{Q_{cold}}{Q_{hot}},
from
\Delta U = Q_{hot} - Q_{cold} - Work
Which is cylci and this delta u = 0.
I am going the ruight way about this problem?
Many thanks,
TFM