chemstudent123
- 13
- 0
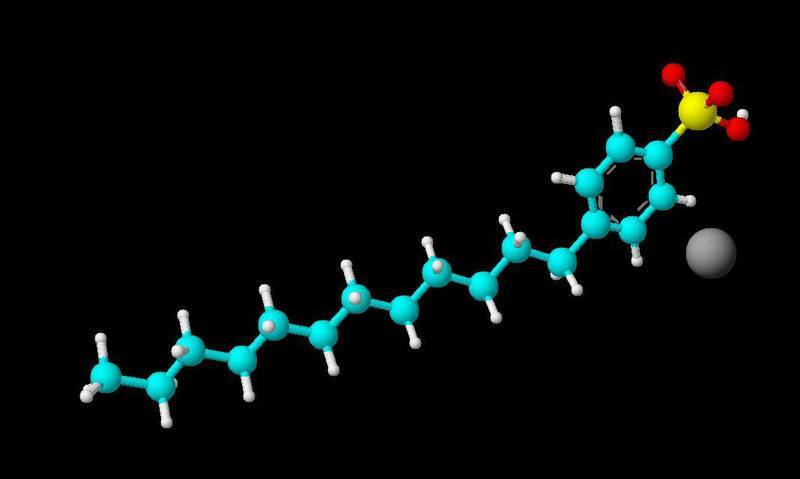
I have to write a report on the bonds of the following element sodium doecylbenzene sulfonate. The problem is in the pic (see pic) the sodium doesn't have a bond to anything is this an ionic bond? Plz help I'm extremely confused