Adesh
- 735
- 191
Summary:: I'm always unable to find the products of a chemical reaction. No Matter how much concept I study I can't ever get products from reasoning.
Here is the question:
What is the major product of this reaction?
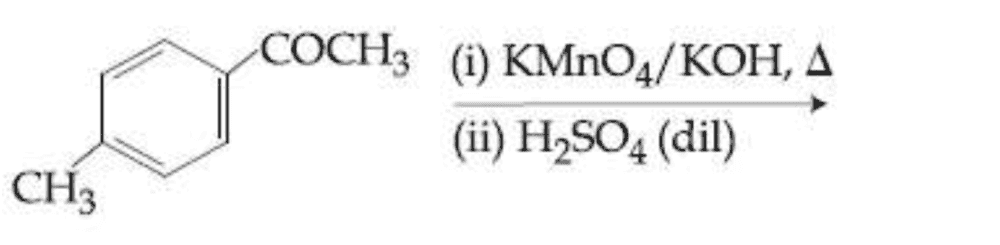 .
.
OPTIONS :
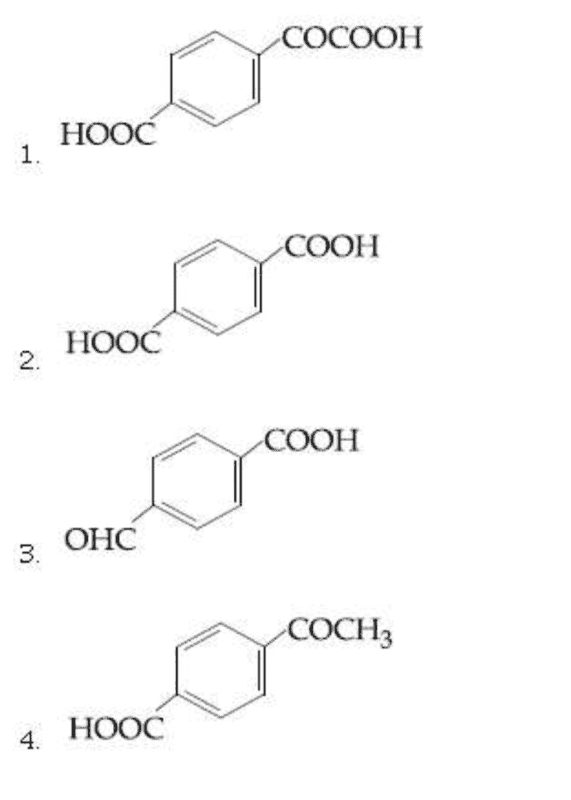 MY ATTEMPT :
MY ATTEMPT :
First of all I can see that in question I have a benzene ring to which a methyl group is attached, on the other side we have a ketone. The structure of the molecule in question can be drawn as:
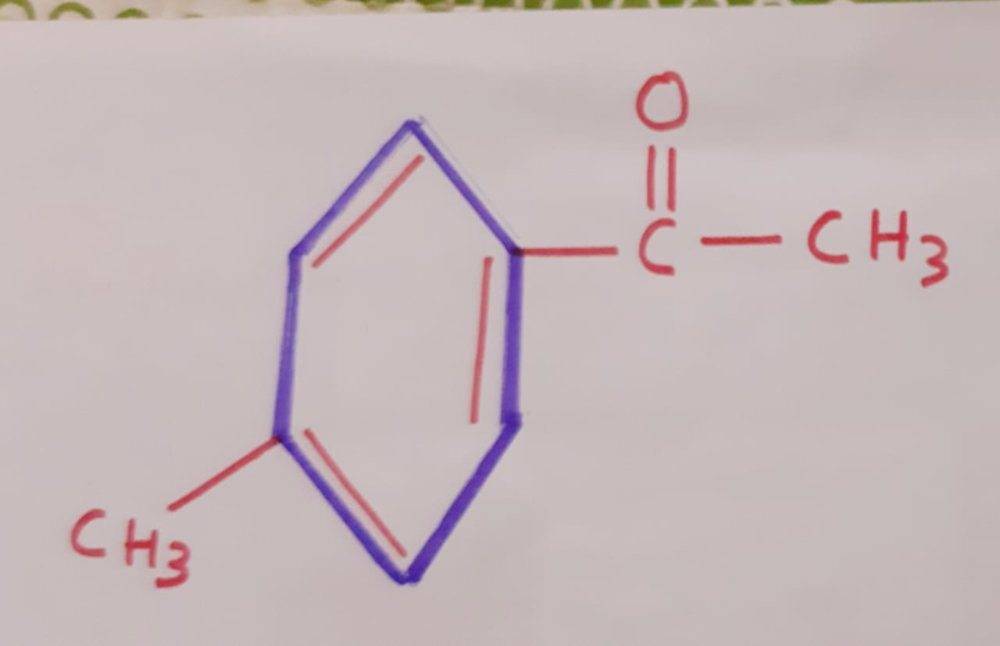 . Now, I know that oxidation (with strong oxidizing agents) of alcohols, aldehydes and ketones results in carboxylic acid. Therefore, I treat the above molecule (a ketone group) with strong oxidizing agent KMnO_4 we would would get something like this
. Now, I know that oxidation (with strong oxidizing agents) of alcohols, aldehydes and ketones results in carboxylic acid. Therefore, I treat the above molecule (a ketone group) with strong oxidizing agent KMnO_4 we would would get something like this
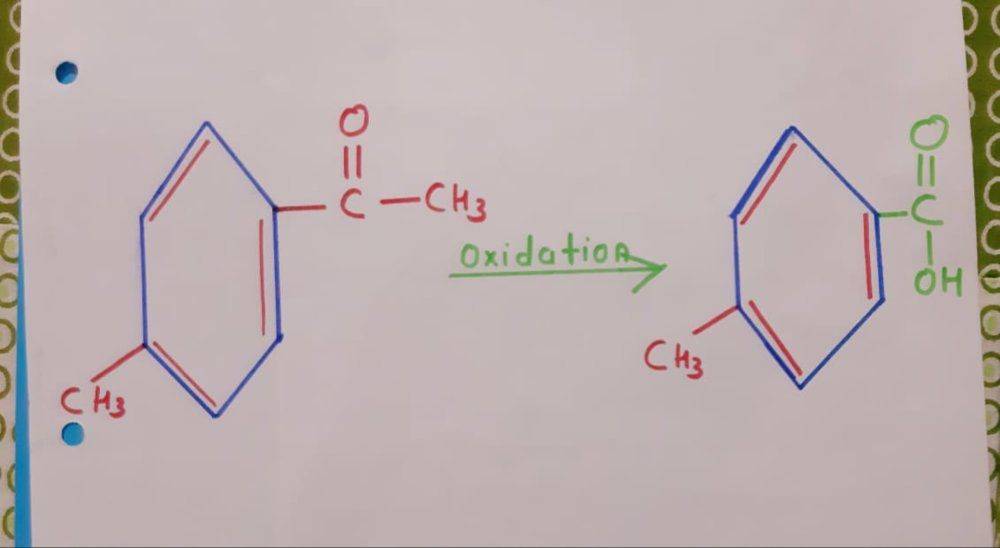 . (I really don't know what effect would KOH have on our molecule because as per my knowledge OH^- is a nucleophile and hence attacks reagents like alkyl chloride, benzyl chloride. In this case I have no idea what would happen).
. (I really don't know what effect would KOH have on our molecule because as per my knowledge OH^- is a nucleophile and hence attacks reagents like alkyl chloride, benzyl chloride. In this case I have no idea what would happen).
This much being done, now we treat our carboxylic acid with sulfuric acid and according to me there will be no reaction. There are chances that benzene ring (which contains \pi electrons) can act as a base, but seeing the options I have to eradicate this idea.
The methyl group CH_3 which is attached to our starting molecule is, according to my studies, is not reactive. Alkenes undergo oxidation to form alkynes or alcohol (I may be wrong here, I said alkynes because removal of hydrogen from alkenes ,i.e. oxidation, would result in alkynes or the addition of OH , i.e. addition of oxygen to carbon, would result in alkyl alcohols). Alkanes can only be combusted,
Thank you. Any help will be much appreciated.
P.S. :- I highly apologize for posting the images, but I have no other option. If you can suggest me any other way of drawing molecules (using latex or something) then please do suggest me.
Here is the question:
What is the major product of this reaction?
OPTIONS :
First of all I can see that in question I have a benzene ring to which a methyl group is attached, on the other side we have a ketone. The structure of the molecule in question can be drawn as:
This much being done, now we treat our carboxylic acid with sulfuric acid and according to me there will be no reaction. There are chances that benzene ring (which contains \pi electrons) can act as a base, but seeing the options I have to eradicate this idea.
The methyl group CH_3 which is attached to our starting molecule is, according to my studies, is not reactive. Alkenes undergo oxidation to form alkynes or alcohol (I may be wrong here, I said alkynes because removal of hydrogen from alkenes ,i.e. oxidation, would result in alkynes or the addition of OH , i.e. addition of oxygen to carbon, would result in alkyl alcohols). Alkanes can only be combusted,
.Alkyl halides are almost never prepared by direct halogenation of alkanes. From the standpoint of synthesis in tha laboratory, an alkane is a dead end.
-Organic Chemistry by Morrison and Boyd
Thank you. Any help will be much appreciated.
P.S. :- I highly apologize for posting the images, but I have no other option. If you can suggest me any other way of drawing molecules (using latex or something) then please do suggest me.
