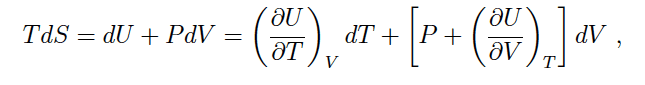SUMMARY
The discussion focuses on the expansion of internal energy (U) in terms of temperature (T) and volume (V) when expressing the differential of entropy (dS). The mathematical relationship is defined by the equation dU/dT(V) + dU/dV(T), which represents the total differential of U with respect to the coordinates (T, V). This expansion does not alter the meaning of the expression, as it adheres to the principles of thermodynamics. The conversation also touches on the number of intensive properties necessary to define the state of a single-phase material with constant composition.
PREREQUISITES
- Understanding of thermodynamic principles
- Familiarity with the concepts of internal energy (U), temperature (T), and volume (V)
- Knowledge of differential calculus in the context of thermodynamics
- Basic grasp of intensive and extensive properties in materials science
NEXT STEPS
- Study the derivation of the first law of thermodynamics
- Learn about the Maxwell relations in thermodynamics
- Explore the concept of state functions and their differentials
- Investigate the role of intensive properties in phase equilibrium
USEFUL FOR
Students and professionals in thermodynamics, materials science researchers, and anyone interested in the mathematical foundations of energy transformations in physical systems.