You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Internal: Definition and 991 Discussions
An internality is the long-term benefit or cost to an individual that they do not consider when making the decision to consume a good or service. One way this is related to behavioral economics is by means of the concept of hyperbolic discounting, in which immediate consequences of a decision are disproportionately weighed compared to the future consequences. A potential cause is lack of access to full information regarding the associated costs and benefits prior to consumption. This contrasts with traditional economic theory, which makes the assumption that individuals are rational decision makers who take all personal costs into account when paying for goods and services.One example of a positive internality is the long run effect of exercising, if these are not taken into account when deciding whether to exercise. Future benefits that an individual may not take into consideration include a diminished risk of heart disease and higher bone density. A common example of a potential negative internality is the effect of smoking cigarettes on those who smoke. For the effect of secondhand smoke, see externality. Statistically, 80% of smokers want to quit, and 54% of people who are serious about quitting fail in a week or less. This implies that they do not act in their long-term best interest due to short-term discomfort, also known as a self-control problem. If the demand for cigarettes has a high price elasticity of demand, which evidence seems to suggest, the government can combat the negative internality by raising taxes. It is important to note that elasticity might change based on location and knowledge about the harmful health effects of smoking. In traditional economic theory, a tax diminishes the welfare of the poor because the tax burden shifts to low-income communities, as fewer can afford the good (cigarettes), and horizontal equity (economics) is distorted. However, behavioral economic theory suggests that the tax is not regressive if low-income communities have higher (healthcare) costs and more price sensitivity than individuals with higher incomes. Taxes imposed to combat internalities are most effective when they target a specific good. A tax on junk food could apply to a large variety of goods that are widely consumed, and the cost of the tax might be perceived as more detrimental than beneficial for society. A major issue with creating effective legislature against negative internalities is that the tax imposed should only reflect the cost that individuals do not factor into their consumption decisions. The difficulty in measuring individual knowledge is an obstacle to developing new policies. Another point of concern is that the group benefitting from the tax, such as smokers who want to quit, must be sizable enough to offset any backlash from tobacco companies and lobbyists.
In the following graphs, D' and S' are the demand and supply curves if producers and consumers take all external costs (EC) into consideration. The tax attempting to prevent the internality should be set equal to the difference between D and D' at the optimal quantity, which is the unmeasured internal cost (IC).
View More On Wikipedia.org
In the following graphs, D' and S' are the demand and supply curves if producers and consumers take all external costs (EC) into consideration. The tax attempting to prevent the internality should be set equal to the difference between D and D' at the optimal quantity, which is the unmeasured internal cost (IC).
View More On Wikipedia.org
-
D
Calculating current with and without internal resistance
Homework Statement Calculate the magnitudes and directions of the currents in each of the 3 resistors in the circuit above, if... (a) ...both batteries have zero internal resistance. (b) ...each battery has an internal resistance of 1.0 Ω. Homework Equations I1 = I2 + I3 (Node Law)The...- ddobre
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
Enthelpy & Internal Energy Change relation with Cp & Cv
Homework Statement Match the following Given : Processes do not include chemical reactions. Assume CP,m and CV,m are independent of temperature for given substance and consider only pressure-volume work in given all processes. Homework Equations ΔU = Q - W ΔH = ΔU +...- cooldudeachyut
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
P
Finite difference conjugate heat xfer: internal flow
Hi. I have been trying to solve this problem that has been keeping me up at night for a coupe weeks at least. If anyone can help me, I would be greatly appreciated. Hot air enters a cylindrical duct. The duct has some R-value and radiation and convection is being accounted for on the outside...- pm272
- Thread
- Replies: 6
- Forum: Mechanical Engineering
-
D
Batteries in series, parallel, and internal resistance
Homework Statement Three identical batteries are first connected in parallel to a resistor. The power dissipated by the resistor is measured to be P. After that, the batteries are connected to the same resistor in series and the dissipated power is measured to be 4P (four times larger than for...- ddobre
- Thread
- Replies: 24
- Forum: Introductory Physics Homework Help
-

What is the main source of Earth's internal heat?
I've been wondering about this question for some time now. There are the following two contributors: ~~~~~~~~~~~~~~ 1. Heat left over from the planet's initial formation. In the early 19th century Lord Kelvin estimated the temperature based on a homogenous sphere of uniform initial temperature...- Facial
- Thread
- Replies: 25
- Forum: Earth Sciences
-
P
Solving internal flow exiting temp w/ conjugate HT BCs.
Hi. There is a problem that I have been working on and I seem to be getting somewhat unrealistic results. Can anyone critique my modeling method? Problem: Heated air enters a duct of length L at temp T_h. The outside of the thin walled duct will have convection and radiation both being...- pm272
- Thread
- Replies: 4
- Forum: Mechanical Engineering
-

Question about measuring the internal resistance of power supplies
In measuring the internal resistance of a power supply, why is a resistor with low resistance used?- yubson
- Thread
- Replies: 8
- Forum: Electrical Engineering
-

Calculation of the change in internal energy of water
Homework Statement If water vapor is assumed to be a perfect gas, molar enthalpy change for vaporization of 1 mol of water at 1 bar and 100 C is 41 kJ/mol . Calculate the internal energy, when 1 mol of water is vaporized at 1 bar pressure and 100 C. Homework Equations $$\Delta U = \Delta H =...- NoahCygnus
- Thread
- Replies: 19
- Forum: Biology and Chemistry Homework Help
-
E
Why is internal energy not a function of pressure?
I'm reading a book on thermal physics and the author says this: "In general, the internal energy will be a function of temperature and volume, so that we can write U =U(T,V) " How do we know this intuitively and how do we know that internal energy is not a function of pressure as well?- eprparadox
- Thread
- Replies: 6
- Forum: Mechanics
-

B Why do objects have internal energy?
From what I understand, the molecules/atoms that make up an object are in constant, random motion. Do electromagnetic forces cause this motion or what is the cause of it? If so, why would net electromagnetic forces exist between "neutral" atoms?- Vectronix
- Thread
- Replies: 2
- Forum: Other Physics Topics
-
R
Internal Resistance of a Car Battery
Homework Statement An automobile battery has a terminal voltage of 12.8 V with no load. When the starting motor (which draws 90A) is running, the terminal voltage drops to 11.0 V. What is the internal resistance of the battery? Homework Equations V= EMF- Ir The Attempt at a Solution I think...- Reliquo
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
A
What values of WT should I use for internal radiation dose calculations?
Homework Statement Question attached. I want to check what values of WT I should use. Homework Equations Effective dose = WT*WR*WT,R The Attempt at a Solution For the neutron whole-body dose I have WT = 1. For the ingested 90Sr and 131I, I also have WT = 1. Are these correct or does...- adamworth
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
B
Transient heat transfer in a cylinder with internal heating
hi, I met a problem about heat transfer in cylinder, if you can help, I will appreciate it. The question is simple. I want to know the transient heat distribution in a cylinder with internal heating(constant temperature not constant flux). The boundary conditions comprises two constant...- Bruce
- Thread
- Replies: 27
- Forum: General Engineering
-
T
Calculate the Internal Energy of Monoatomic Gas
Homework Statement Re-arranging the equations of potential (internal) energy in a monoatomic gas, i get this differential equation: \gamma (dV/V ) + (dP/P) = 0. where V is volume and P is pressure. I have to integrate it. Homework EquationsThe Attempt at a Solution I tried, but i wasnt able...- themagiciant95
- Thread
-
- Tags
- Energy Gas Internal Internal energy
- Replies: 12
- Forum: Advanced Physics Homework Help
-
A
Thermodynamics- Piston problem, pressure, internal energy
Homework Statement Homework Equations F= ma P= F/A The Attempt at a Solution a). Assuming the piston is in equilibrium, I'm applying Newton's second law F=ma=0 Equals zero because it is not moving Note: P=F/A which I rearrange to solve for the force for F= PA. This represents the...- Ashley1nOnly
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
C
Internal energy and total heat of universe
Hello. I am starting to learn about thermodynamics. (i'm going to use lower case "d" for delta) Energy is neither created nor destroyed. So dU = 0 for the universe as a whole. If the universe is constantly expanding, then it must be doing work on the vacuum around it, right? So W is a...- CuriousBanker
- Thread
- Replies: 3
- Forum: Thermodynamics
-
F
Java Java Saving Data In File Internal
Hello I am trying to read data from one activity, save it to a file... and when open another activity I will be able to read that file(by opening it). I searched for tutorials, all they are showing on the same activity. I figured out how the code works.. but I'm not able to understand what will... -

Internal energy during the expansion of a gas?
I have question regarding the (W = ∫Pdv) formula for the work done during the expansion of an ideal gas and the change in internal energy during the process. If we were to have a gas enclosed inside an insulated cylinder with a movable piston at one end with cross sectional area "a", I...- Pericles98
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-
W
Internal quantum efficiency (IQE)
Homework Statement The IQE is defined as (photons emitted from active region ##s^{-1}##) / electrons injected across the junction. Derive an expression for this quantity, then find the injected electron concentration ##n## which maximises it. Homework EquationsThe Attempt at a Solution We've...- whatisreality
- Thread
-
- Tags
- Efficiency Internal Quantum
- Replies: 1
- Forum: Advanced Physics Homework Help
-
B
External Weak Product is the Internal Weak Product of…
Homework Statement Let ##\{G_i \mid i \in I\}## be a family of groups, then ##\prod^w G_i##, the external weak direct product, is the internal weak direct product of the subgroups ##\{i_k(G_k) \mid k \in I\}##, where ##i_k : G_k \to \prod G_i## is the canonical embedding. Homework...- Bashyboy
- Thread
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
B
Internal Direct Product
Homework Statement Let ##H, K, N## be nontrivial normal subgroups of a group ##G## and suppose that ##G = H \times K##. Prove that ##N## is in the center of ##G## or ##N## intersects one of ##H,K## nontrivially Homework EquationsThe Attempt at a Solution I presume that ##G = H \times K##...- Bashyboy
- Thread
- Replies: 7
- Forum: Calculus and Beyond Homework Help
-

How to find internal energy with constant temperature?
Homework Statement since specific heat c changes with temperature, but its treated as a constant in the heat formula, so that means that heat formula Q=mc(T2-T1) is just an approximation? correct? I see some texts define heat as Heat, q, is thermal energy transferred from a hotter system to a...- EastWindBreaks
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-

How to find internal energy with constant temperature?
since specific heat c changes with temperature, but its treated as a constant in the heat formula, so that means that heat formula Q=mc(T2-T1) is just an approximation? correct? I see some texts define heat as Heat, q, is thermal energy transferred from a hotter system to a cooler system that...- EastWindBreaks
- Thread
- Replies: 1
- Forum: Thermodynamics
-

How to get the internal energy?
Homework Statement Homework Equations The Attempt at a Solution v2=vf+x*vfg x =(0.4458-0.001067)/(0.71813-0.001067) =0.61973 u2=uf+x*ufg =535.08+0.61973*(2001.8-535.08) =1444.05kJ/kg ==> far different from solution! whats wrong with my attempt? thanks- yecko
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
Internal energy of an ideal gas as a function of pressure?
Assuming all gases in the combustion reaction of benzoic acid (C6H5COOH) behave ideally, what is the "exact" change in internal energy? The context in which this question is being asked is after a calorimetry experiment. For all the intents and purposes of calorimetry, the change in internal...- Jacob Dale
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-

I Internal energy of any ideal gas
The internal energy of monoatomic ideal gas is due to the kinetic energy of the molecules. Using Boltzmann Maxwell distribution, it is calculated that the kinetic energy due to translational motion of gas molecules of an ideal gas depends only on the temperature. In case of monoatomic gas, since...- Pushoam
- Thread
- Replies: 2
- Forum: Other Physics Topics
-
P
(Just Released) New internal combustion engine technology
please share with others- poe
- Thread
- Replies: 3
- Forum: Mechanical Engineering
-
I
EMF and internal resistance laboratory work
1. The problem statement I did a laboratory work in which I had to find power and internal resistance. I used an ordinary battery so it's closed circuit. I did all tasks except writing a hypothesis and conclusion I can't really think of it. I don't know what to write since I just measure using...- isorounded
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
I
Electric power and internal resistance
Homework Statement Today I did a laboratory work in which I had to find power and internal resistance. I used an ordinary battery so it's closed circuit. I did all tasks except writing a hypothesis and conclusion I can't really think of it. I don't know what to write since I just measure using...- isorounded
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
C
Inelastic collision and the sum of internal forces
Homework Statement Suppose I have a system which contains two bodies m1 and m2 with initial velocities v1 and v2 , respecitvely. they hurl toward each other and make an inelastic collision. such that they are now one body of mass m1 + m2 I know that the difference in momentum is...- CGandC
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
P
I TIR (total internal reflection) in terms of quantum world
how total internal reflection of light takes place in terms of quantum world?- Pritamstar
- Thread
- Replies: 3
- Forum: Quantum Physics
-

Unraveling the Mystery of EMF Internal Resistance
I'm having a difficult time understanding why the internal resistance of the EMF source is disregarded in this problem. 1. Homework Statement You are asked to determine the resistance per meter of a long piece of wire. You have a battery, a voltmeter, and an ammeter. You put the leads from the...- Ryaners
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
C
Solving for the internal forces and reactions
Homework Statement I'm having a hard time finding the reactions at the supports and the force exerted by the spring. Given: 20 lbs 25 lbs 35 lbs k = 200 lb/ft lo = 2.5 ft members are 4 ft longHomework Equations ΣM ∑Fy = 0 ∑Fx = 0 The Attempt at a Solution I tried solving for the reactions...- chunchunmaru
- Thread
- Replies: 9
- Forum: Engineering and Comp Sci Homework Help
-
R
Finding the internal resistance of battery
Homework Statement I measured a battery rated at 1.5V(ε) and get 0.9V out and want to calculate the internal resistance (r). So, I model the battery as an ideal voltage source and a resistor in parallel. I know the voltmeter has a resistance of 1kΩ (R). How do I calculate the internal...- rmiller70015
- Thread
- Replies: 10
- Forum: Introductory Physics Homework Help
-
L
I Understanding Internal Energy: Kinetic Theory and the Law of Equipartition
Internal energy at a specific state can't be calculated but by kinetic theory of gases and law of equipartition of energy Average kinetic energy is directly proportional to temperature.And for an ideal gas internal energy is due to kinetic energy only for an ideal gas potential energy can be...- LEELA PRATHAP KUMAR
- Thread
- Replies: 15
- Forum: Classical Physics
-
E
Internal energy of an ideal gas as a function of temperature
Homework Statement Two containers hold an ideal gas at the same temperature and pressure. Both containers hold the same type of gas but container B has twice the volume of container A. The internal energy of the gas in container B is (a) twice that for container A (b) the same as that for...- eprparadox
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
G
Rotational kinetic energy decreases, internal increases?
If rotational kinetic energy of a closed system decreases, another form of energy must increase for the conservation of energy of a closed system. We assume this system (a person in rotation) has these forms of energy: ΔE=ΔK (only rotational around an axis) + ΔUi (internal) + ΔEt (thermal) with...- Gian_ni
- Thread
- Replies: 4
- Forum: Mechanics
-
T
Electric motors vs internal combustion engines
On average, which has higher power to weight ratio between the two? So on average, if both weighed the same, which one is going to have more power?- Tabaristiio
- Thread
- Replies: 9
- Forum: General Engineering
-
H
Integral constant for internal energy of an ionic liquid
Integral constant for internal energy of ionic liquid I have a question, and I will be really grateful if someone helps me. I have a polynomial equation for internal energy which I calculated by integration an equation of state formula, which is based on density. But, because I calculated this...- hosein
- Thread
- Replies: 20
- Forum: Chemistry
-
H
Integral constant for internal energy of ionic liquid
Integral constant for internal energy of ionic liquid I have a question, and I will be really grateful if someone helps me. I have a polynomial equation for internal energy which I calculated by integration an equation of state formula, which is based on density. But, because I calculated this... -
C
Internal resistance of a battery cell
if a cell is running down, there would be a constant drop in p.d. across the cell, does the internal resistance of the cell increase or decrease?- Cici2017
- Thread
- Replies: 2
- Forum: Electromagnetism
-
B
B Internal energy of compressed gas
Hi, I've been reading about compressed air energy storage and keep coming across that in 300 bar containers the achievable energy is 0.1MJ/L. Is this 0.1MJ/L of the volume of the air it is compressed to or of the total L of air that was initially used? (E.g If 1500L is compressed to 300 bar...- Bhope69199
- Thread
- Replies: 8
- Forum: Classical Physics
-
N
Internal pressure due to vessel collapse
Hey All, I have a plastic vessel fully filled filled with an incompressible fluid (Water), at some time this vessel is impacted and crushed on one side (say 5% of the initial volume is lost). Only, I know the volume isn't lost, the fluid (being incompressible) will exert pressure on all... -
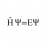
Finding compressibility from given internal Energy function
Hi everyone! 1. Homework Statement Given is a function for the internal energy: ##U(T,V)=Vu(T)## Asked is to derive the entropy balance equation. In order to do so i need to find the "isothermal and adiabatic compressibility": $$\kappa_{T}=-\frac{1}{V}\left(\frac{\partial V}{\partial...- H Psi equal E Psi
- Thread
- Replies: 7
- Forum: Advanced Physics Homework Help
-
T
Potentiometer and EMFs with internal resistance
Homework Statement In a potentiometer 99cm of wire of resistance 99ohms is connected with a battery of 50V and 1ohm internal resistance.Find emf of battery giving zero deflection for a length of 13cm. Homework Equations Sadly,the formula I know is only for ideal emfs,which states that: If two...- Tanishq Nandan
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-

Internal Energy of an Ideal gas related to Molar specific heat
Homework Statement Please look at the below images which is the derivation of the relation between the internal energy of an ideal gas and the molar specific heat at constant volume. (Snaps taken from Fundamentals of Physics Textbook by David Halliday, Jearl Walker, and Robert Resnick) As...- SciencyBoi
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
T
Potential difference of an emf with internal resistance
Homework Statement A battery of emf 2V and internal resistance 0.1ohm is being charged with a current of 5 ampere. In what direction will the current flow inside the battery?What is the potential difference between the two terminals of the battery? Homework Equations If a battery of emf E and...- Tanishq Nandan
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
M
Internal Energy of virial expansion
Hello, I have some trouble understanding the virial expansion of the ideal gas. 1. Homework Statement I have given the state equation: $$ pV = N k_b T \left(1+\frac{A\left(T\right)}{V}\right) $$Homework Equations [/B] and a hint how to calculate the caloric equation of state $$...- Mikhail_MR
- Thread
- Replies: 10
- Forum: Advanced Physics Homework Help
-

Change of internal energy of an ideal gas
Homework Statement Homework Equations Δu = ∫ [(a-Ru)+bT+cT^2+dT^3]dT The Attempt at a Solution The answer of 6447kJ/kmol is given but I am struggling to get to this answer after integrating the above formula and inserting the given values. Firstly would the integral of...- DevonZA
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
S
Understanding Internal Energy Changes in Phases of Water
Homework Statement Sketch a diagram of internal energy (y-axis) versus temperature in the range from -10°C to +112°C to indicate how energy would change for a fixed quantity of water in its three phrases. Label and explain the main features of the variation. Homework Equations The Attempt at...- SpiraRoam
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help