You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Molecular: Definition and 580 Discussions
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.
In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. This violates the definition that a molecule contain two or more atoms, since the noble gases are individual atoms.A molecule may be homonuclear, that is, it consists of atoms of one chemical element, as with two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, as with water (two hydrogen atoms and one oxygen atom; H2O).
Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considered single molecules.Molecules as components of matter are common. They also make up most of the oceans and atmosphere. Most organic substances are molecules. The substances of life are molecules, e.g. proteins, the amino acids they are made of, the nucleic acids (DNA & RNA), sugars, carbohydrates, fats, and vitamins. The nutrient minerals ordinarily are not molecules, e.g. iron sulfate.
However, the majority of familiar solid substances on Earth are not made of molecules. These include all of the minerals that make up the substance of the Earth, soil, dirt, sand, clay, pebbles, rocks, boulders, bedrock, the molten interior, and the core of the Earth. All of these contain many chemical bonds, but are not made of identifiable molecules.
No typical molecule can be defined for salts nor for covalent crystals, although these are often composed of repeating unit cells that extend either in a plane, e.g. graphene; or three-dimensionally e.g. diamond, quartz, sodium chloride. The theme of repeated unit-cellular-structure also holds for most metals which are condensed phases with metallic bonding. Thus solid metals are not made of molecules.
In glasses, which are solids that exist in a vitreous disordered state, the atoms are held together by chemical bonds with no presence of any definable molecule, nor any of the regularity of repeating unit-cellular-structure that characterizes salts, covalent crystals, and metals.
View More On Wikipedia.org
In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. This violates the definition that a molecule contain two or more atoms, since the noble gases are individual atoms.A molecule may be homonuclear, that is, it consists of atoms of one chemical element, as with two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, as with water (two hydrogen atoms and one oxygen atom; H2O).
Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considered single molecules.Molecules as components of matter are common. They also make up most of the oceans and atmosphere. Most organic substances are molecules. The substances of life are molecules, e.g. proteins, the amino acids they are made of, the nucleic acids (DNA & RNA), sugars, carbohydrates, fats, and vitamins. The nutrient minerals ordinarily are not molecules, e.g. iron sulfate.
However, the majority of familiar solid substances on Earth are not made of molecules. These include all of the minerals that make up the substance of the Earth, soil, dirt, sand, clay, pebbles, rocks, boulders, bedrock, the molten interior, and the core of the Earth. All of these contain many chemical bonds, but are not made of identifiable molecules.
No typical molecule can be defined for salts nor for covalent crystals, although these are often composed of repeating unit cells that extend either in a plane, e.g. graphene; or three-dimensionally e.g. diamond, quartz, sodium chloride. The theme of repeated unit-cellular-structure also holds for most metals which are condensed phases with metallic bonding. Thus solid metals are not made of molecules.
In glasses, which are solids that exist in a vitreous disordered state, the atoms are held together by chemical bonds with no presence of any definable molecule, nor any of the regularity of repeating unit-cellular-structure that characterizes salts, covalent crystals, and metals.
View More On Wikipedia.org
-
B
[PoM] Rotational and vibrational heat capacity
Hi guis, i need your help... 1. Homework Statement Evaluate the rotational and vibrational contributions to the heat capacity of a gas of DBr (D=deuterium, Br=mixture at 50% of 79Br and 81Br) at 380 K temperature, knowing that the bond distance is 1.41 Å and the vibration frequency of 1H79Br...- BRN
- Thread
- Replies: 12
- Forum: Advanced Physics Homework Help
-

Anti-hydrogen molecular spectra?
I'm not a molecular physicist, so my speculations are bound to be somewhat naive. I'm only hoping to initiate some discussion around the subject, which I thought might be interesting to all. Now that the electronic absorption spectrum of the atomic anti-hydrogen 1s->2s electronic transition...- Mark Harder
- Thread
- Replies: 7
- Forum: Mechanics
-

I Molecular Hamiltonian - Ammonia
Hello everybody, The general expression of molecular Hamiltonian operator for any molecule is: $$\hat{H} = \hat{T}_n+\hat{T}_e+\hat{V}_{ee}+\hat{V}_{nn}+\hat{V}_{en}+\hat{f}_{spin-orbit} $$ where: ##\hat{T}## correspond to kinetic energy operator ##\hat{V}## correspond to potential energy...- Konte
- Thread
-
- Tags
- Ammonia Hamiltonian Molecular
- Replies: 2
- Forum: Quantum Physics
-

A Determining molecular resonance frequencies
I have been searching for ways to calculate resonance frequencies of complex molecules. I know that doing so is extremely complex, especially if that molecule contains many elements, but perhaps it could be feasible to target a specific component of a large molecule, such as a nucleus in a cell...- Cloud Wolf
- Thread
- Replies: 2
- Forum: Quantum Physics
-
E
Standard corrections to GFE for uni and bi molecular rxns
Hello everyone. I have a weird question regarding the calculation of free energy changes in a multi-step mechanism. Now, if you have a bunch of calculated free energies in gas phase at 1 atm (say, from an electronic structure program) and you want to model in solvent, you need to get them in a...- Einstein Mcfly
- Thread
- Replies: 2
- Forum: Chemistry
-

Dot between the molecular formula of compounds
I have seen many instances of molecular formulas of two or more compounds with a dot between two adjacent molecular formulas like CuSO4.10H20, CaCO3.MgCO3, K2SO4.Al2(SO4)3.24H20 and so on. What does the dot indicate? -
L
Chemistry Chemistry: Molecular Iodine and Atomic Iodine
So I just had a question about calculating moles atomic iodine when you have the moles of molecular iodine. In the chemical equation IO3-(aq) + I-(aq) + H+(aq) = I2(aq) + H2O(l) Would the moles of atomic iodine be equal to the moles of the molecular iodine? or would the moles of atomic iodine...- Ly444999
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

A Can a molecular dynamics simulation enter a limit cycle?
In my rough understanding Molecular Dynamics using Classical Newtonian mechanics is a 6N dimensional non linear system. 6N dimension because you have 3 position vectors and 3 momentum vectors for each N particles. Nonlinearity because of the terms in force fields. In principle this system can...- dexterdev
- Thread
- Replies: 2
- Forum: Atomic and Condensed Matter
-
J
A What is the most complex structure you can simulate at home?
Let's say you have a good personal computer with these characteristics: i7-6700K, 32GB RAM, 256GB SSD, 2 x GTX 970 4GB And let's assume you can run your algorithms, software... for 24 hours. I wonder, if I would be calculating the orbital structure of an hydrogen atom with many electrons...- jonjacson
- Thread
- Replies: 3
- Forum: Atomic and Condensed Matter
-

Nobel prize: molecular machines
The Nobel prize in chemistry for 2016 has been awarded "for the design and synthesis of molecular machines." These are fascinating constructions made up of just a few molecules, so they are the smallest machines possible. It is far from trivial to get these things working, especially since...- DrClaude
- Thread
-
- Tags
- Machines Molecular Nobel prize
- Replies: 7
- Forum: Chemistry
-

Misc. Create Molecular Models with Balls & Links - Pros & Cons
I want to make my own set of molecular models with balls representing atoms and links representing bonds. Anyone knows of a good software to do this? As for the physical model, what bond angles, bond lengths and ball sizes should I create? I could for example leave atoms as round balls and...- Edwina Lee
- Thread
- Replies: 2
- Forum: DIY Projects
-
A
Molecular Biologist with interest in Quantun Biology
Hello everyone! I am really glad to be part of this group. I hold a Bachelor of Science with Honors in Molecular Biology from the University of Sydney. During the past 10 years, I built a strong career in the business side of research, working as a technical sales consultant, and despite the...- Ana Luisa
- Thread
- Replies: 1
- Forum: New Member Introductions
-
T
Chemistry Molecular formula of organic compound
Homework Statement Homework EquationsThe Attempt at a Solution Firstly I tried to find the empirical formula: C : H : O 65.2 : 8.75 : 26.05 (mass) 5.43 : 8.75 : 1.628 (mol) 3.34 : 5.37 : 1 10 : 16 : 3 C10H16O3 This gives a molecular mass of 184. Now with the structure: It has to...- TT0
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-

Electromagnetism and injury -- physical contact at the molecular level....
So here is my question, and maybe I am not asking it right, but here we go: If electromagnetism prevents me from actually touching anything at a quantum level, how is it that I can get cut by a knife, or get a road rash falling off my bike? If the negative field of electrons that surround...- Nate0331
- Thread
- Replies: 3
- Forum: Electromagnetism
-
T
How does the distance between molecules affect temperature?
Homework Statement According to the kinetic molecular theory: as the distance between the molecules increases, the temperature of a material decreasesthe rise in temperature of a material occurs because the molecules lose kinetic energywhen the molecules within a gas collide they lose energy...- TT0
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
N
Electrons energy levels do not change with molecular bonding
So if energy levels, or eV of electrons, do not change with molecular bonding, how are electrons influencing each other.? In glass, when individual atoms of silicon, sodium, and calcium come together, to form glass, the molecular bonding does not change the energy levels of the electrons for...- Nicholas Ham
- Thread
- Replies: 13
- Forum: Chemistry
-

Stochastic dynamics and Angular velocity of a molecular motor
Homework Statement Homework Equations Langevin equation (I included all the equations in the next section.[/B]The Attempt at a Solution I do not know how I can proceed from this point. I'm stuck since I have no information on the drag coefficient. Maybe my approach is wrong, and there may...- kev931210
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
V
What home based solvent might dissolve unknown molecular particles?
What home based solvent might dissolve an unknown electron microscope seen insoluble particles ? There are some sort of unknown insoluble particles in our water. Does anyone know of any home remedy acid solution (baking soda and vinegar) that might dissolve these particles ? Please keep in... -
I
The molecular switch from welcoming to attacking a pathogen?
I watched many videos and read bunch of answers about this, but I was having a hard time finding an answer to this one point: What is the exact moment an organism realizes that it is under an attack? I understand that there might be a few different types of ways a body can react too. So far...- icakeov
- Thread
-
- Tags
- Antibodies Cell Molecular Switch
- Replies: 6
- Forum: Biology and Medical
-

Chemistry Determine Weight Percent given molecular weight....
Homework Statement So for my homework assignment in 'Materials Science and Engineering', We were given the following problem. 1. Li and Ra both are both BCC metals. For this problem assume they form a complete solid solution (even though the very large difference in their atomic size tell us...- hdp12
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Does B2 Have a Sigma Molecular Orbital?
Guys I studied Molecular orbital theory based on wave mechanics in lower level as i don't know what is wave mechanics so i saw molecular orbital diagram of boron di atomic molecule in which there are two pi molecular orbitals so my first question was that there must be first a sigma molecular...- Karan Punjabi
- Thread
- Replies: 3
- Forum: Chemistry
-
A
Can Heun's Method Accurately Predict Small Oscillations of a Nitrogen Atom?
Homework Statement Use the Heun method to compute the period of small oscillations about the equilibrium position of a nitrogen atom. xi = 1.1 Um = 7.37 x0 = 1.2 alpha = 2.287 m = 2.325e-26 Homework Equations [/B] U(x) = Um((1-e^(-alpha(x-x0)))^2 - 1) The Attempt at a Solution I was told to...- akaPaul
- Thread
-
- Tags
- Method Molecular Oscilation Spring
- Replies: 3
- Forum: Engineering and Comp Sci Homework Help
-

Heat as Electromagnetic and Molecular Kinetics
Consider the image below Does it emit all 3 types of heat in the form of UV, IR and light? At what threshold does light start to appear and what colors should it illuminate? Hypothetically, say I have an induction furnace and is capable of setting frequencies to about 700THz, will it be able to...- Ronie Bayron
- Thread
- Replies: 1
- Forum: Electromagnetism
-
H
Problem with molecular vibrations
In IR spectroscopy, bonds vibrate and therefore there are moving charges and usually a changing dipole. If accelerating charges cause EM waves to be given off due to the changing electric field then shouldn't the vibrating bond be constantly emitting photons and therefore losing energy in a...- hinobi
- Thread
-
- Tags
- Molecular Vibrations
- Replies: 9
- Forum: Atomic and Condensed Matter
-
C
How to Keep Up with Molecular Orbitals for Intro Chemistry
I am a professional math and science tutor, primarily focused on math and physics. I used to tutor organic chemistry but gave it up because I didn't want to study orgo an hour per week for the rest of my life. I like general chemistry and have no trouble with most topics, but the way of...- Cruikshank
- Thread
- Replies: 6
- Forum: STEM Educators and Teaching
-
M
Number of molecular hits from air onto a surface
Homework Statement Compute the average number of molecular hits, per unit time, experienced by a square inch of surface exposed to air, under normal conditions. Assume air is a mixture composed of 80% N2, 20% O2, both of which are assumed to be ideal gases. You will have to perform angular...- mr-serendip
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
B
Chemistry "Simple molecular structure do not conduct electricity" H2O?
Homework Statement Book says "Substances with a simple molecular structure do not conduct electricity. This is because they do not have any free electrons or an overall electric charge". Homework EquationsThe Attempt at a Solution I think the statement is a generalization because water is a...- Barclay
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
B
Chemistry Understanding Simple Molecular Structures in Chemistry
Homework Statement What are simple molecular structures?Homework EquationsThe Attempt at a Solution Simple molecular structures consist of molecules containing fixed numbers of atoms joined by strong covalent bonds. I'm getting confused with all the different structures in chemistry. How...- Barclay
- Thread
-
- Tags
- Molecular Structures
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
R
Relating excited state molecular emission to chemical reaction
.. reaction. This may seem a nonsensicle question but I'll go ahead anyway. Say if I have a process that has an emission spectra dominated by excited state molecular emission, can I relate this in any way to the amount / number of chemical reactions occurring? Thanks for any help -
A
What is an oscillating molecular dipole defined as?
Is an oscillating dipole of a molecule the periodic motion of the atoms in the molecule (the oscillating back and forth of a more negatively charged atom and more positively charged atom), or, is an oscillating molecular dipole just oscillations in electron density? I just can't get a clear...- apeters
- Thread
-
- Tags
- Dipole Molecular Oscillating
- Replies: 2
- Forum: Atomic and Condensed Matter
-
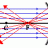
Average Speed for Maxwell's Distribution of Molecular Speed
Using the Maxwell-Boltzmann equation above, there is an example in my book (Giancoli 4th edition p. 481) where they use this to find the average velocity. I understand that it would just be the sum of all the speeds of the molecules divided by the number of molecules. But then I'm having...- RaulTheUCSCSlug
- Thread
- Replies: 2
- Forum: Electromagnetism
-
S
Specific Heat and its relevance to Molecular Configuration
Hello, this is one of the problems in my laboratory manual and it has been killing me. I've been working on this all week. It's due tonight, so hopefully it's not too late... 1. Homework Statement Explain why specific heat is "specific", and how it gives a relative indication of molecular...- Satonam
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Moles, Molecular mass, Number of Molecules Im lost.
So I am taking the second semester of intro physics and Moles,Molecular mass, Number of Molecules is killing me. I am not really able to solve my homework because I can never get these quantities down. I asked my professor for help during offie hours, however he said he teaches physics not... -

Chemistry Molecular Energy With Relation to Number of Molecules
Homework Statement Which solid-line curve most accurately represents the distribution of molecular energies in a gas at 500 K if the dotted-line curve represents the corresponding distribution for the same gas at 300 K? Homework Equations - The Attempt at a Solution Not sure how this...- Priyadarshini
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Schools Chances of Getting Into Graduate School Ph.D Programs
I am a rising senior at a small liberal arts college, with an incredibly small (and therefore unrecognized) physics program. I am seeking advice regarding which Ph.D programs are within my reach. I plan on applying to AMO (Atomic, Molecular, and Optics) Ph.D programs with the intention of... -
Y
Study molecular conformation using optical spectroscopy?
I want to study the molecular conformation of conjugated polyelectrolyte (CPE) using optical spectroscopy. Obviously a lot of people have used optical spectroscopy to study the structure of conjugated polymers, including this conjugated polyelectrolyte. However, they did in when the CPE is in...- Yinxiao Li
- Thread
- Replies: 1
- Forum: Chemistry
-
K
Impact of molecular bonding on therm. neutron cross-sections
Hi everyone. Most of what I've read on thermal neutron cross sections talks only about what isotope makes up the neutron target. Likewise, all of the cross sections listed on Sigma: http://www.nndc.bnl.gov/sigma/index.jsp?as=9&lib=endfb7.1&nsub=10 ... are broken down just by isotope. However... -
S
Molecular orientation and volume
In the process for orienting molecular chains, for example dyneema, does the volume of the polymer change? In the process for creating dyneema, the fibers are drawn, heated and elongated. Would this change the volume of fiber? I know the density for dyneema is listed at .97. The density of...- Samson4
- Thread
-
- Tags
- Molecular Orientation Volume
- Replies: 1
- Forum: Other Physics Topics
-
V
Calculating required amount of molecular sieves for PSA?
Does anyone know how to properly calculate the amount of molecular sieves needed for a pressure swing adsorption system? I have searched application notes, books, and of course google, and while I find the basics of how they are constructed I cannot find any information on the quantity...- Voltux
- Thread
-
- Tags
- Molecular
- Replies: 1
- Forum: Materials and Chemical Engineering
-
H
Chemistry Molecular Solid vs Network Covalent Solids
Homework Statement I'm having trouble figuring out if a solid is a molecular solid or network covalent solid. Classify the following as a network covalent solid or a molecular solid: 1. CO2 2. SiO2 Homework Equations Molecular solid held together by intermolecular forces Covalent network... -

What Does Molecular Orbital Theory Say About the Overlap of Atomic Orbitals?
Hey! So I'm a little fuzzy on my understanding of MO theory. One question I had on my study guide said that according to MO theory, overlap of two s atomic orbitals produces _________. I know the answer is one bonding molecular orbitals and one antibonding molecular orbital. Why is this? Also I... -
M
Molecular Modeling: Geometry Optimization, Vibration Analyses & More
Hi there! Is there an online, hopefully free resource (preferably textbook) that details the process of molecular modeling? Specifically, computationally performing geometry optimizations, vibrational analyses, calculating Raman spectra, detailing the theory behind Hartree-Fock, DFT, post-HF... -
B
Molecular Modeling of Fluid Mechanics
As I've been reading up on fluid mechanics recently, I've discovered several different molecular models which attempt to explain heterogeneous fluid flow from a molecular level. Classical fluid mechanics assumes that hard body approximation- that atoms are hard spheres knocking into each other...- Ben Johnson
- Thread
- Replies: 2
- Forum: Beyond the Standard Models
-

Molecular Hydrogen Ionization
The ionization energy of H2 is 1488 kJ/mol. How strong of a DC electrostatic field would be need to ionize H2 molecules all at a distance 'd' from the source of the field? What I am asking is how to convert the known unit (joule) into a measure of an electrostatic field since electric fields are...- Misha Kuznetsov
- Thread
-
- Tags
- Hydrogen Ionization Molecular
- Replies: 6
- Forum: Mechanics
-

Deriving Ideal Gas using Molecular Flux Equation
So basically I was wondering whether it's possible to get the expression of ideal gas using molecular flux equation which is ##\phi = \frac{1}{4}\bar{v}n##. The derivation should be straightforward. I need to get the expression of pressure. Because the flux by definition already gives the rate... -
L
Molecular Hydrogen absorption spectrum
Is it true, as someone says, that molecular hydrogen in its fundamental state don't (almost?) absorb in the frequencis of the Balmer series of atomic hydrogen emission spectrum? Thanks. -- lightarrow- lightarrow
- Thread
- Replies: 2
- Forum: Atomic and Condensed Matter
-
W
How Do Orbital Interactions and Sigma Bonds Work in Methane?
Hello all I have an exam coming up this week but there's something that I don't understand: Let's take methane, for example. If I understand it correctly, the bonding orbitals of carbon are all hybridized to sp³ orbitals and overlap with the 1s orbital of four H atoms. Does the orbital of...- Watari
- Thread
- Replies: 1
- Forum: Atomic and Condensed Matter
-

What is the relationship between atomic structure and molecular orbital theory?
Plzz explain me molecular orbital& relation beetween atomic- akashpandey
- Thread
- Replies: 6
- Forum: Quantum Physics