Emission Definition and 546 Threads
-

How do you find the temperature of an HII region?
I first approached this problem with the idea that I could try to find the temperature of the HII region given that we already know the background temperature. Still, I am stuck on finding the region's temperature. A second approach was to try to find if the cloud is optically thick, which...- Danielk010
- Thread
- Absorption Emission Spectral lines
- Replies: 0
- Forum: Advanced Physics Homework Help
-

Help with CE-102 test -- failing with emissions too high
I'm seeking assistance and recommendations for CE-102 testing (MIL-STD 461). When I power on my unit, the emission levels significantly exceed the limit. Since the unit is already designed, I cannot make any internal modifications. Could you please suggest potential causes for this issue and...- core7916
- Thread
- Emission Testing
- Replies: 16
- Forum: Electrical Engineering
-

Undergrad Distance at redshift z=0.666
According to the ΛCDM model, the light we receive today from a galaxy located at a distance of 8.10 Gyr took 6.31 Gyr to reach us and has a redshift of ##z=0.666## (cosmological calculator). At what distance today is a galaxy that in the future receives with a redshift of ##z=0.666## a signal...- Jaime Rudas
- Thread
- Emission Signal
- Replies: 37
- Forum: Cosmology
-

Undergrad Battery life on VERY fast moving object
Accepted thinking is that time slows as speed increases, relative to a non-moving reference frame. But supposing that the object approaching C is a ship in rotation around the earth, and on that ship is a laser mounted on a gimbal such that the laser is always pivoting and trained on exactly...- davidjoe
- Thread
- Battery Emission Laser
- Replies: 128
- Forum: Special and General Relativity
-
N
Undergrad Equations of motion of an electron emitted from a surface
Homework Statement: Real world application of freshman physics Relevant Equations: TBD This is not a homework question, this is relevant to my work. It seems simple enough (introductory) but I keep running into problems. An electron is emitted from an surface (material is irrelevant, could... -

Undergrad Phonon emission : Discrete event or a process with inner detail?
If I understand correctly, when an electron drops to a lower energy state and emits a phoTon, this is a discrete or "atomic" event in the sense that it can't be meaningfully broken down in terms of more detailed sub-processes or interactions. Now in the case of phoNon emission, it is also...- Swamp Thing
- Thread
- Discrete Emission Phonon
- Replies: 2
- Forum: Quantum Physics
-

Rate of EM emission based on surface characteristics
I would think the black painted surface is- druscilla
- Thread
- Emission Surface
- Replies: 6
- Forum: Introductory Physics Homework Help
-
S
Decrease in Psti and Plti values with transposed Pst and Plt (@LV) to HV?
Do Psti and Plti values decrease when the measured Pst and Plt (@LV) is transposed to HV?- samirbista71
- Thread
- Emission Limits
- Replies: 3
- Forum: Electrical Engineering
-
T
Undergrad Fourier Transform of Photon Emission Hamiltonian
Hey all, I just wanted to double check my logic behind getting the Fourier Transform of the following Hamiltonian: $$H(x) = \frac{ie\hbar}{mc}A(x)\cdot\nabla_{x}$$ where $$A(x) = \sqrt{\frac{2\pi\hbar c^2}{\omega L^3}}\left(a_{p}\epsilon_{p} e^{i(p\cdot x)} + a_{p}^{\dagger}\epsilon_{p}...- thatboi
- Thread
- Emission Fourier Fourier transform Hamiltonian Photon Photon emission Transform
- Replies: 4
- Forum: Quantum Physics
-

Undergrad Do Emission and Absorption Spectra Match? A Non-Physics Minded Tourist's Guide
Basic stuff. Do emission and absorption spectra match? If so, why wouldn't hot stellar atmospheres exhibit both, cancelling? I'm a tourist...not physics minded..- Paul Howard A
- Thread
- Absorption Emission Match Spectra
- Replies: 4
- Forum: Astronomy and Astrophysics
-

Spectral lines in the emission spectrum for an electron at n= 3
Since there is only one excited electron, it could come from n=3 to n =1directly or n=3 to n =2 and then n=2 to =1. Hence, there could be one or two lines depending upon the path taken by electron. Is this right?- Pushoam
- Thread
- Electron Emission Emission spectrum Lines Spectral lines Spectrum
- Replies: 2
- Forum: Advanced Physics Homework Help
-

Undergrad Double-Slit Experiment: Does rate of photon emission matter?
Hi there! High school physics teacher hoping to pick the brains of people who know more than I do here. I'm curious whether the rate of photon emission has any noticeable effect on the diffraction pattern generated by the double-slit experiment. To be clear: I understand a diffraction pattern...- Mr Fallspring
- Thread
- Diffraction pattern Double-slit Double-slit experiment Emission Experiment Matter Photon Photon emission Rate
- Replies: 16
- Forum: Quantum Physics
-
B
Undergrad Emission of light from incandescence of metals
a. We know metals emit EM radiation upon heating or electric current. I'd like to understand more fundamentally how this phenomenon takes place, on the basis of the basis of band structure, and which electrons are involved ? b. Classically, charges emit radiation when accelarating or...- bentzy
- Thread
- Band structure Current Emission Light Radiation
- Replies: 5
- Forum: Atomic and Condensed Matter
-
S
Undergrad Can there be some kind of photon emission caused by space expansion?
Are there any kind of observed and experimentally verified processes or mechanisms where photon emission occurs and which are directly cause by spacetime expansion in some way? -

Question on Emission Spectra/Spectral Series | Atomic Physics
(I need help with the 2nd part as I can answer the theory part properly). For E=4 eV we can find the wavelength of emitted photon. E= 4 eV = 6.4087e-19 J Using E= hc/λ we get λ=310 nm (approx) My doubt is that this should fall in the Balmer Series but we know that the lowest wavelength value...- warhammer
- Thread
- Atomic Atomic physics Atomic spectra Emission Physics Series
- Replies: 26
- Forum: Introductory Physics Homework Help
-

Graduate Cause of spontaneous emission?
I am reading this chapter 3 from the book called The Quantum Vacuum by P.Milonni.(Attached in the pdf, look at chapter 3.2 Spontaneous emission)There they say that spontaneous emission is due to both quantum fluctuations and radiation reaction. They say the transitions induced by the quantum...- sol47739
- Thread
- Cause Emission Laser cavity Photon emission Quantum mechanics Spontaneous Spontaneous emission
- Replies: 15
- Forum: Quantum Physics
-
M
Undergrad Absorption and emission spectroscopy of atoms
Hello ! As I understand it, the different isotopes of the same atom have a slightly different spectroscopic absorption and emission where, for example, Deuterium absorbs slightly shorter wavelengths than Protium. My question is if two isotopes of different atoms, for example Tritium and Helium...- MartinG
- Thread
- Absorption Atoms Emission Spectroscopy
- Replies: 3
- Forum: Atomic and Condensed Matter
-

Graduate Question about stimulated Raman emission
I have read a paper states that "Stimulated Raman emission relies on damping of the phonon field that is much greater than for that of the optical Stokes field". But I cannot understand this, since all the materials I read do not state this. Can anyone explain it intuitively?- ure227922
- Thread
- Emission Raman Stimulated
- Replies: 2
- Forum: Quantum Physics
-
D
Undergrad What determines the time between atomic absorption and emission of photons?
What determines the time between atomic absorption and emission of photons? Is there a correlation to blackbody radiation?- dsaun777
- Thread
- Absorption Atomic Emission Photons Time
- Replies: 19
- Forum: Atomic and Condensed Matter
-
D
Undergrad Find the interference function for different emission modes
Homework Statement:: Find the interference function ##I(\delta)## where The emission is analyze by a Michelson interferometer. Relevant Equations:: ##I(\delta) = \frac{1}{2} \int_{-\infty}^{\infty} G(k) r^{ik \delta} dk## ##I(\vec{r}) = I_1 + I_i + 2 \sqrt(I_1 I_i) cos (k\delta)## I have 5...- DragonBlight
- Thread
- Emission Function Interference Michelson interferometer Modes Optical Spectral lines
- Replies: 2
- Forum: Optics
-
D
Graduate Possible Ways to reduce Electron field Emission threshold
Hello there, i wanted to ask if anyone knows a process or mechanism, that reduces the electric field that is requiered to tunnel an electron. When i use the work function of 4 eV (Aluminum) i get with Schottky-Nordheim approach a field of 870 kV/mm to tunnel an electron. Measurements tho just...- domovinavi
- Thread
- Electron Emission Field Threshold
- Replies: 1
- Forum: Quantum Physics
-
C
Graduate Absorption and emission spectrum in quantum optics
The emission spectrum or resonance fluorescence for a quantum dot, atom or defect center are discussed in many quantum optics textbook, for example see "Quantum Optics" by Marlan O. Scully and M. Suhail Zubairy Chapter 10 , "Quantum Optics" by D. F. Walls and Gerard J. Milburn Chapter 10 and...- Cedric Chia
- Thread
- Absorption Absorption spectrum Emission Emission spectrum Optics Quantum Quantum optics Spectrum
- Replies: 4
- Forum: Quantum Physics
-
J
Very High Standard Deviation in Excitation Emission Matrix Measurement
Hi, I obtain really high standard deviations in Excitation-Emission Spectra mainly for the phenolic compounds in olive oil (Em: 290-350nm). Method: I weigh 0.05g of olive oil and dilute it up to 25ml with cyclohexane to remain in the range of linearity for absorbance measurements to correct... -
L
Undergrad Observing Emission Spectra from Computer Screens
Hi! I am doing some simple observations of different light sources with a simple DIY spectroscope. When I look at a computer screen I see what I believe to be an emission spectrum due to the dark spectrum with emission lines on it. Is this correct? And why does a computer screen emit an...- Luxdot
- Thread
- Computer Emission Spectra
- Replies: 2
- Forum: Electromagnetism
-

Graduate Spontaneous and stimulated emission in Planck's radiation law
Hello, Einstein introduced stimulated emission (along with spontaneous emission and absorption) to derive Planck's radiation law using his A and B coefficients in his 1917 paper. My question is, is it possible to separate the Planck radiation spectrum into a fraction that is spontaneous...- dbabic
- Thread
- Emission Law Radiation Spontaneous Stimulated Stimulated emission
- Replies: 5
- Forum: Quantum Physics
-
C
Undergrad Assumptions for blackbody spectra vs. emission spectra vs. absorption spectra
Hi there, I am a physical oceanographer teaching an introductory undergraduate Earth science class that has a unit on astronomy. I have a physics undergraduate background, took a few astronomy classes at the undergraduate level back in the day, and did a bit of undergraduate research in...- curious_ocean
- Thread
- Absorption Assumptions Blackbody Emission Spectra
- Replies: 8
- Forum: Astronomy and Astrophysics
-
N
High School Do emission nebula glow because of ionised or excited electrons?
I'm trying to figure out why emission nebulae glow. I read various sites such as a NASA website explaining why they shine; 'The massive stars embedded within the nebula give off enormous amounts of ultraviolet radiation, ionizing the gas and causing it to shine.' The Britanica article on...- Nathi ORea
- Thread
- Electrons Emission Excited Glow Nebula
- Replies: 4
- Forum: Astronomy and Astrophysics
-
F
De-excitation of a moving atom with photon emission
The information I have are the following: ##p^\mu=(E, p, 0, 0)## ##p'^\mu=(E', p'\cos\beta, -p'\sin\beta,0)## ##k^\mu=\tilde{E}(1, \cos\alpha, \sin\alpha, 0)## Where: ##E=\sqrt{M^2+p^2}## ##E'=\sqrt{m^2+p'^2}## Using the conservation of the four-momentum ##p^\mu=p'^\mu+k^\mu##...- Frostman
- Thread
- Atom Conservation of energy Emission Four momentum Photon Photon emission Special relativity
- Replies: 6
- Forum: Advanced Physics Homework Help
-

Chemistry Why Does UV Light Have More Energy Than Visible Light in an Emission Spectrum?
The difference in energy between these two lines is that in the ultraviolet spectrum line, there is more energy because it has a shorter wavelength compared to the visible spectrum line as shown in figure 1.1 According to the Niels Bohr's model of the atom(figure 1.7) and figure 1.1, the least...- muissi97
- Thread
- Element Emission Emission spectrum Spectrum
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
High School Prevalence of nuclear decays accompanied by gamma emission
Some alpha or beta decays produce an excited daughter nucleus, which typically immediately emits one or more gama rays to reach a ground state. This is the case for beta decay of Co-60 or Na-24 for example. While the table of cobalt isotopes on Wikipedia mentions the gamma emission, the one for...- Petr Matas
- Thread
- Alpha decay Beta decay Emission Gamma Nuclear
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-
P
Undergrad Why are emission spectra of stars rarely shown?
According to this link you just have to anlayse the light that isn't coming from a place on the star that has a light the source directly behind it e.g wouldn't looking at light from the outer edge of star give you an emission spectrum? http://www.thestargarden.co.uk/Spectral-lines.html- pkc111
- Thread
- Emission Spectra Stars
- Replies: 5
- Forum: Astronomy and Astrophysics
-
K
Undergrad Stimulated emission in harmonic oscillator
Hello! Is stimulated emission possible for a harmonic oscillator (HO) i.e. you send a quanta of light at the right energy, and you end up with 2 quantas and the HO one energy level lower (as you would have in a 2 level system, like an atom)?- kelly0303
- Thread
- Emission Harmonic Harmonic oscillator Oscillator Stimulated Stimulated emission
- Replies: 4
- Forum: Quantum Physics
-
K
Undergrad Confused about spontaneous emission
Hello! I thought that in spontaneous emission (say for an atom with 2 energy levels) we have the electron in the excited state and then it decays to the ground state emitting a photon at the resonance frequency. However I saw the attached figure, which introduces Mollow triplet. I understand the...- kelly0303
- Thread
- Confused Emission Spontaneous Spontaneous emission
- Replies: 6
- Forum: Atomic and Condensed Matter
-
I
Undergrad Relation between blackbody radiation and spontaneous emission
I'm wondering what the relationship between blackbody radiation and spontaneous emission is. As far as I know, there are three sources of EM radiation - thermal radiation, oscillating dipole (multipole?), and LASER. And it seems like light emission from an atom can be separated into two...- IcedCoffee
- Thread
- Blackbody Blackbody radiation Electro dynamics Emission Radiation Relation Spontaneous Spontaneous emission
- Replies: 6
- Forum: Quantum Physics
-
R
Undergrad Qualitative description of stimulated emission & population inversion
I'm having trouble understanding stimulated emission and population inversion, and how they work together to make a laser work. I pretty much need this explained completely. 1. Spontaneous emission, they say, is when an atom absorbs and then later emits a photon. Isn't that just regular...- rtareen
- Thread
- Emission Inversion Laser population Stimulated Stimulated emission
- Replies: 18
- Forum: Quantum Physics
-

Undergrad On the Rydberg Constant and the Emission Lines
With regard to Rutherford's atomic model, and Rydberg's discovery in general for the hydrogen distribution lines, what does Rydberg's constant physically mean? Its unit is m ^ -1, as if it were a rate, but it was not clear to me its physical meaning. And why does it grow with atomic mass...- Gabrielmonteiro
- Thread
- Atomic model Constant Emission Lines Modern physics Quantu physics Rutherford
- Replies: 6
- Forum: Quantum Physics
-
R
Undergrad How does this experimental result show photon emission?
First I'll explain my understanding, because I'm not very confident in it. The main point is that the electrons are ejected and then accelerated to a very high kinetic energy. Then they start smashing into the anode. Most will go through a series of collisions before completely stopping, so that...- rtareen
- Thread
- Emission Experimental Photon Photon emission Photons
- Replies: 11
- Forum: Quantum Physics
-
M
High School Redshifted Photon Emission vs Transport: Magnitude of Gravitational Redshift
I am considering the magnitude of the gravitational redshift and I look at the process of a photon leaving an atom from the Sun. I am asking whether the processes in the atom, viewed as a clock, would lead us to conclude that the emitted photon, at the time of emission, would itself be...- Mickey1
- Thread
- Emission Photon Photon emission Transport
- Replies: 10
- Forum: Special and General Relativity
-
F
Electron-positron annihilation, photon emission angle
I consider the laboratory system. The four momentums in this reference system are respectively: ##p^\mu = \big(\sqrt{|p|^2+m^2}, 0, 0, |p| \big)## ##p'^\mu= \big(m, 0, 0, 0 \big)## ##k^\mu = E\big(1, 0, 1, 0\big)## ##k'^\mu = E'\big(1, 0, -\sin \varphi, \cos \varphi \big)## I used conservation...- Frostman
- Thread
- Angle Annihilation Emission Photon Photon emission
- Replies: 13
- Forum: Advanced Physics Homework Help
-
J
Undergrad Are all processes CPT symmetric like measurement, stimulated emission?
https://en.wikipedia.org/wiki/CPT_symmetry says "CPT theorem says that CPT symmetry holds for all physical phenomena" - e.g. we could imagine decomposition of given phenomena into Feynman diagrams and apply CPT symmetry to all of them. However, for some o processes such reversibility seems...- Jarek 31
- Thread
- Cpt symmetry Emission Measurement Reversibility Stimulated Stimulated emission Symmetric
- Replies: 8
- Forum: Quantum Physics
-
T
1420 MHz--- the emission frequency of cold hydrogen gas
I recently finished reading Paul Davies book The Eerie Silence, which is a book about the SETI (Search for ExtraTerrestrial Intelligence) project. In The Eerie Silence, Davies says that scientists using radio telescopes to search for radio messages from space aliens set their radio telescopes...- timmeister37
- Thread
- Cold Emission Frequency Gas Hydrogen
- Replies: 30
- Forum: Chemistry
-

Graduate A mathematical description of the physics behind Aurora?
Maybe a bit of an odd question (not really sure where it would belong on this site to be honest), but I was wondering if anyone can explain, or at least knows of a source that explains in a quantitative way, the physics behind aurora? Now I've seen websites like this that discuss conceptually...- dykuma
- Thread
- Aurora Emission Fluorescence Lightning Mathematical Physics
- Replies: 3
- Forum: Beyond the Standard Models
-
P
High School 1-photon emission possible from electron-positron annihilation?
I was reading about electron-positron annihilation. Typically it results in two photons, each with an energy of 511 keV, that go shooting out in opposite directions. But I read that in some instances three photons can result. Electrons have an intrinsic spin of ½, while photons have a spin of 1...- Puffer Fish
- Thread
- Annihilation Beam Emission Positron
- Replies: 23
- Forum: Quantum Physics
-
M
Photon emission in electronic transitions
How would you explain, on a basic level, why only one photon (as opposed to two, three...) is emitted when an electron in an atom changes its energy level? This is for students with only introductory Physics background.- MuonMinus
- Thread
- Electronic Emission Photon Photon emission
- Replies: 2
- Forum: STEM Educators and Teaching
-
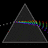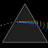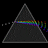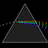
Hydrogen Emission Spectrum and Electron Energy Levels
1. The 4th line from the left, being the aqua blue line, corresponds to a wavelength of 486 nm, as blue light has a wavelength in the range 450-495 nm. 2. This is where I am having the most difficulty, I have tried to answer the question comprehensively but I am not satisfied with my answer. In...- AN630078
- Thread
- Electron Emission Emission spectrum Energy Energy levels Hydrogen Levels Spectrum
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Undergrad How to read this decay sheet (gamma emission after beta decay)
I was looking at the decay scheme (https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html) of ##^{112}Ag## which ##\beta##-decays to ##^{112}Cd##. ##Cd## is most likely left in an excited states, so it decays to its ground state by ##\gamma##-emission. As you can see there are tons of...- dRic2
- Thread
- Beta Beta decay Decay Emission
- Replies: 4
- Forum: High Energy, Nuclear, Particle Physics
-
K
High School Can spontaneous emission be considered a thermodynamic process?
I realize that nothing causes an excited atom to emit a photon, and that it's a random process. But someone was asking me about why energized systems in general tend to lose their energy to the environment and move toward equilibrium. I mentioned that an inflated balloon, given a hole, will tend...- Karl Coryat
- Thread
- Emission Process Spontaneous Spontaneous emission Thermodynamic
- Replies: 4
- Forum: Quantum Physics
-

Optical depth using Bremsstahlung emission coefficient
Equations I think may be relevant:- Kayla Martin
- Thread
- Assignment Astronomy Astrophysics Coefficient Depth Emission Optical Physics
- Replies: 3
- Forum: Advanced Physics Homework Help
-
K
Undergrad The life cycle of a star and the bell shaped energy emission curve
Do all stars in their life cycle (t) emit energy (E) that follow a bell shape curve? If yes, is the curve symmetrical always? How is this related to nuclear and thermal time scale?- kinchit bihani
- Thread
- Bell Curve Cycle Emission Energy Life Star
- Replies: 11
- Forum: Astronomy and Astrophysics
-
O
High School Emission from a coated cathode and tunneling
I am wondering about an exercise exam question (it isn't homework): "at low temperatures (<2000 K), thermionic emission of a tungsten cathode depends on tunneling. By coating the tungsten with a suitable substance, the emission by tunneling can be greatly increased. Question: which two...- Orthoceras
- Thread
- Cathode Emission Tunneling
- Replies: 2
- Forum: Quantum Physics