Andrew Mason said:
I was not saying necessarily that the point where dQ/dV = 0 is the intersection with the isotherm. I was just saying that up to that point there has been net heat flow into the system and after that point net heat flow is negative (out of the system).
I am not sure where dQ/dV = 0 but I agree that this is what you want to find. Up to that point, dQ/dV>0 (heat flow is in) and after, dQ/dV<0 (heat flow is out). From the first law, dQ/dV = dU/dV + dW/dV = dU/dV + P (W is the work done by the gas). So you want to find the point on the line where dQ/dV = 0 i.e. where: dU/dV = -P.
AM
vela said:
You want to find the point where 0 = dQ/dV = dU/dV ± dW/dV (not sure what sign convention you're using).
View attachment 195546
I may be mistaken. but I am pretty sure that positive is work done on the gas, and negative is work done by the gas.
Positive is heat absorbed and negative is heat removed.
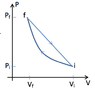
A thermodynamic cycle consists of only 2 processes and uses a monatomic ambient gas, First, the monatominc gas is compressed adiabatically from the initial pressure Pi=1 bar to Pf=5 bar starting at temperature Ti=300 K The initial volume of the gas before the compression is Vi=10 liters. In the second process gas expands along straight line on the PV diagram. Find the Efficiency.
Here's what I have got so far:
Pi=10^5 Pa
Vi = .01 m^3
ni = .4 mol
Ti = 300 K
Pf = 5*10^5
Vf = .0038 m^3
nf = .4 mol
Tf = 571.6 K
Wtotal = 1354.2 J (adiabat) + -1860J (linear) = -505.8 J
Qi->f = 0
Qf->i = 505.8 J = Qh + Qc
Part of this is the sum of heat absorbed and heat given off. this doesn't make a whole lot of sense to me because the process absorbs more heat than it exhausts, but the temperature drops...
I found the eq for the linear expansion to be ##-6.45*10^7V + 745161##
knowing this and I found the temperature during expansion to be ##T = \frac{-6.45*10^7V^2 + 745161V}{.4*8.31}##
and the max temperature would be 647.308 K at V = .00577644 m^3
Seems strange that the temperature would rise during expansion first... but it isn't for long. .0038 -> .0058 is pretty short between .0038 and .01
What do I do. I know this is a lot to ask. I don't think I learned what you are talking about.