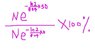Ukitake Jyuushirou
- 124
- 0
Iodine-131 is used in treatment of thyroid disorder. This isotope has a half life of 8.04 days, what percentage of an initial sample is left after 30 days?
having done, the initial calculations by taking 30 divided by 8.04 to find how many hl will have passed i take the number and i take the mass number divided by the amount of hl that has passed times 100 but my answers dun match the answer in the book.
where did i go wrong?
having done, the initial calculations by taking 30 divided by 8.04 to find how many hl will have passed i take the number and i take the mass number divided by the amount of hl that has passed times 100 but my answers dun match the answer in the book.
where did i go wrong?
Last edited: